Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0T8BA
|
||||
| Former ID |
DNC001391
|
||||
| Drug Name |
Sulfonamide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Phase 3 | [521417] | ||
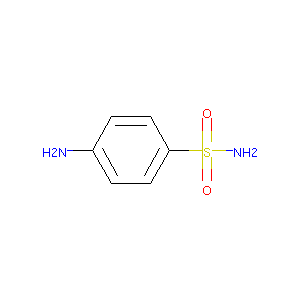
| Structure |
|
Download2D MOL |
|||
| Formula |
C6H8N2O2S
|
||||
| Canonical SMILES |
C1=CC(=CC=C1N)S(=O)(=O)N
|
||||
| InChI |
1S/C6H8N2O2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H2,8,9,10)
|
||||
| InChIKey |
FDDDEECHVMSUSB-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 423136-40-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9661, 73452, 588082, 608543, 866440, 3134404, 4492185, 7885628, 7890374, 7980703, 8149606, 8153275, 10321137, 10531344, 11112170, 11335703, 11360942, 11363383, 11365945, 11368507, 11372371, 11374094, 11376669, 11461914, 11466757, 11467877, 11484567, 11486333, 11488662, 11491221, 11492213, 11494303, 14748191, 16957260, 17389527, 24860368, 24870531, 24899829, 26612000, 26679377, 26697120, 26702537, 26704167, 26747313, 26747314, 29224387, 32447070, 46508306, 46511429, 47193670
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | Dihydropteroate synthetase | Target Info | Binder | [535199] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

