Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0TQ1G
|
||||
| Former ID |
DAP001087
|
||||
| Drug Name |
Rimantadine
|
||||
| Synonyms |
Remantadine; Riamantadine; Rimant; Rimantadina; Rimantadinum; Enamine_005755; Alpha-Methyladamantanemethylamine; Flumadine (TN); Rimantadina [INN-Spanish]; Rimantadine (INN); Rimantadine [INN:BAN]; Rimantadinum [INN-Latin]; Alpha-Methyl-1-adamantanemethylamine; Rimant & .alpha. IFN; Rimantidine & .alpha.IFN; Tricyclo(3.3.1.1^3,7)decane-1-methanamine, .alpha.-methyl-& IFN.alpha; 1-(1-Adamantyl)ethanamine; 1-(1-Adamantyl)ethylamin; 1-(1-adamantyl)-ethylamine; 1-(1-adamantyl)ethylamine; 1-(adamantan-1-yl)ethan-1-amine; 1-(tricyclo[3.3.1.1~3,7~]dec-1-yl)ethanamine; 1-Adamantan-1-yl-ethylamine; 1-Adamantan-1-ylethylamine; 1-tricyclo[3.3.1.1~3,7~]dec-1-ylethanamine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Influenza A infection [ICD10:J10, J11] | Approved | [538530] | ||
| Therapeutic Class |
Antiviral Agents
|
||||
| Company |
Forest Laboratories Inc
|
||||
| Structure |
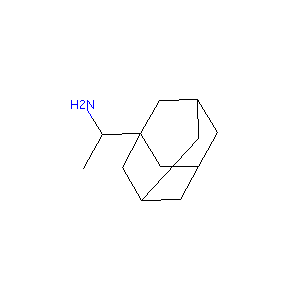
|
Download2D MOL |
|||
| Formula |
C12H21N
|
||||
| Canonical SMILES |
CC(C12CC3CC(C1)CC(C3)C2)N
|
||||
| InChI |
1S/C12H21N/c1-8(13)12-5-9-2-10(6-12)4-11(3-9)7-12/h8-11H,2-7,13H2,1H3
|
||||
| InChIKey |
UBCHPRBFMUDMNC-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 13392-28-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9445, 626310, 643525, 3722269, 3722270, 7379880, 7980519, 8153120, 10506879, 11347986, 11380337, 15120598, 15219656, 16214800, 29215454, 29215455, 29224140, 46505973, 47589624, 47589625, 48416520, 49864987, 57322594, 57820265, 78405535, 92250339, 92309235, 93625279, 96025169, 103250293, 104308193, 104812960, 117395770, 117395954, 117665755, 119525101, 125327867, 125412242, 125539014, 129343015, 132521219, 134224207, 134338344, 134989610, 135692348, 137038244, 139157511, 144021575, 160839516, 160963824
|
||||
| SuperDrug ATC ID |
J05AC02
|
||||
| SuperDrug CAS ID |
cas=013392284
|
||||
| Target and Pathway | |||||
| Target(s) | Influenza A virus M2 protein | Target Info | Inhibitor | [537074] | |
| WikiPathways | Influenza Life Cycle | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

