Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0UZ9U
|
||||
| Former ID |
DNC003573
|
||||
| Drug Name |
PREMETREXED
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Phase 2 | [521700] | ||
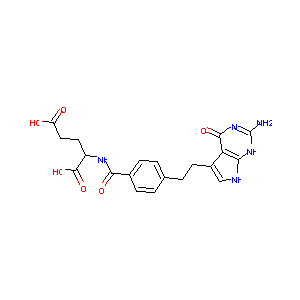
| Structure |
|
Download2D MOL |
|||
| Formula |
C20H21N5O6
|
||||
| Canonical SMILES |
C1=CC(=CC=C1CCC2=CNC3=C2C(=O)N=C(N3)N)C(=O)NC(CCC(=O)O)<br />C(=O)O
|
||||
| InChI |
1S/C20H21N5O6/c21-20-24-16-15(18(29)25-20)12(9-22-16)6-3-10-1-4-11(5-2-10)17(28)23-13(19(30)31)7-8-14(26)27/h1-2,4-5,9,13H,3,6-8H2,(H,23,28)(H,26,27)(H,30,31)(H4,21,22,24,25,29)
|
||||
| InChIKey |
WBXPDJSOTKVWSJ-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Dihydrofolate reductase | Target Info | Inhibitor | [535186] | |
| Thymidylate synthase | Target Info | Inhibitor | [528001] | ||
| BioCyc Pathway | Pyrimidine deoxyribonucleotides biosynthesis from CTP | ||||
| Pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleotides de novo biosynthesis | |||||
| Superpathway of pyrimidine deoxyribonucleoside salvage | |||||
| DTMP de novo biosynthesis (mitochondrial) | |||||
| Pyrimidine deoxyribonucleosides salvage | |||||
| Pathway Interaction Database | E2F transcription factor network | ||||
| PathWhiz Pathway | Folate Metabolism | ||||
| Pterine BiosynthesisPW000160:Pyrimidine Metabolism | |||||
| WikiPathways | Trans-sulfuration and one carbon metabolism | ||||
| Retinoblastoma (RB) in Cancer | |||||
| One Carbon Metabolism | |||||
| Integrated Pancreatic Cancer Pathway | |||||
| miR-targeted genes in muscle cell - TarBase | |||||
| miR-targeted genes in lymphocytes - TarBase | |||||
| miR-targeted genes in leukocytes - TarBase | |||||
| miR-targeted genes in epithelium - TarBase | |||||
| Metabolism of nucleotides | |||||
| Fluoropyrimidine Activity | |||||
| References | |||||
| Ref 528001 | J Med Chem. 2006 Feb 9;49(3):1055-65.Dual inhibitors of thymidylate synthase and dihydrofolate reductase as antitumor agents: design, synthesis, and biological evaluation of classical and nonclassical pyrrolo[2,3-d]pyrimidine antifolates(1). | ||||
| Ref 535186 | Synthesis, antifolate, and antitumor activities of classical and nonclassical 2-amino-4-oxo-5-substituted-pyrrolo[2,3-d]pyrimidines. J Med Chem. 2001 Jun 7;44(12):1993-2003. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

