Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0V3LR
|
||||
| Former ID |
DNC013563
|
||||
| Drug Name |
D-166A
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [529287] | ||
| Structure |
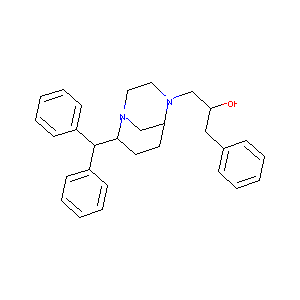
|
Download2D MOL |
|||
| Formula |
C29H34N2O
|
||||
| Canonical SMILES |
C1CC(N2CCN(C1C2)CC(CC3=CC=CC=C3)O)C(C4=CC=CC=C4)C5=CC=C<br />C=C5
|
||||
| InChI |
1S/C29H34N2O/c32-27(20-23-10-4-1-5-11-23)22-30-18-19-31-21-26(30)16-17-28(31)29(24-12-6-2-7-13-24)25-14-8-3-9-15-25/h1-15,26-29,32H,16-22H2/t26-,27+,28-/m0/s1
|
||||
| InChIKey |
WRZZCPUVRXRVCC-IARZGTGTSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Sodium-dependent noradrenaline transporter | Target Info | Inhibitor | [529287] | |
| Sodium-dependent dopamine transporter | Target Info | Inhibitor | [529287] | ||
| Sodium-dependent serotonin transporter | Target Info | Inhibitor | [529287] | ||
| NetPath Pathway | TCR Signaling Pathway | ||||
| PANTHER Pathway | Adrenaline and noradrenaline biosynthesisP00001:Adrenaline and noradrenaline biosynthesis | ||||
| Parkinson disease | |||||
| Dopamine receptor mediated signaling pathwayP04373:5HT1 type receptor mediated signaling pathway | |||||
| 5HT2 type receptor mediated signaling pathway | |||||
| 5HT3 type receptor mediated signaling pathway | |||||
| 5HT4 type receptor mediated signaling pathway | |||||
| Pathway Interaction Database | Alpha-synuclein signaling | ||||
| WikiPathways | Monoamine Transport | ||||
| NRF2 pathway | |||||
| Transport of glucose and other sugars, bile salts and organic acids, metal ions and amine compoundsWP727:Monoamine Transport | |||||
| Dopaminergic Neurogenesis | |||||
| Parkinsons Disease Pathway | |||||
| Transport of glucose and other sugars, bile salts and organic acids, metal ions and amine compounds | |||||
| Neurotransmitter Clearance In The Synaptic CleftWP727:Monoamine Transport | |||||
| SIDS Susceptibility Pathways | |||||
| Synaptic Vesicle Pathway | |||||
| Serotonin Transporter Activity | |||||
| References | |||||
| Ref 529287 | Bioorg Med Chem. 2008 Mar 15;16(6):2769-78. Epub 2008 Jan 11.Further structural optimization of cis-(6-benzhydryl-piperidin-3-yl)-benzylamine and 1,4-diazabicyclo[3.3.1]nonane derivatives by introducing an exocyclic hydroxyl group: interaction with dopamine, serotonin, and norepinephrine transporters. | ||||
| Ref 529287 | Bioorg Med Chem. 2008 Mar 15;16(6):2769-78. Epub 2008 Jan 11.Further structural optimization of cis-(6-benzhydryl-piperidin-3-yl)-benzylamine and 1,4-diazabicyclo[3.3.1]nonane derivatives by introducing an exocyclic hydroxyl group: interaction with dopamine, serotonin, and norepinephrine transporters. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

