Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0V5ZZ
|
||||
| Former ID |
DAP001123
|
||||
| Drug Name |
Propantheline
|
||||
| Synonyms |
Corrigast; Ercorax; Ercotina; Ketaman; Kivatin; Neometantyl; Neopepulsan; Pantas; Pantheline; Pervagal; Probanthine; Probantine; Prodixamon; Propantel; Propanthel; Propanthelinium; Propanthelinum; Bromure de propantheline; Bromuro de proantelina; PROPANTHELINE BROMIDE; Propantelina bromuro; Propantelina bromuro [DCIT];Propanthelini bromidum; P 8891; Bromure de propantheline [INN-French]; Bromuro de proantelina [INN-Spanish]; Pro-Banthine; Pro-Gastron; Propanthelini bromidum [INN-Latin]; SC-3171; Pro-Banthine (TN);Propantheline bromide (JP15/USP/INN); Propantheline bromide [USAN:INN:BAN:JAN]; Diisopropyl(2-hydroxyethyl)methylammonium bromide xanthene-9-carboxylate; Xanthene-9-carboxylic acid, ester with (2-hydroxyethyl)diisopropylmethylammonium bromide; Ammonium, (2-hydroxyethyl)diisopropylmethyl-, xanthene-9-carboxylate (ester); Ammonium, diisopropyl(2-hydroxyethyl)methyl-, bromide, xanthene-9-carboxylate; Methyl-di(propan-2-yl)-[2-(9H-xanthene-9-carbonyloxy)ethyl]azanium; Methyl-di(propan-2-yl)-[2-(9H-xanthene-9-carbonyloxy)ethyl]azanium bromide; N-methyl-N-(1-methylethyl)-N-{2-[(9H-xanthen-9-ylcarbonyl)oxy]ethyl}propan-2-aminium bromide; (2-Hydroxyethyl)diisopropylmethylammonium bromide xanthene-9-carboxylate; (2-Hydroxyethyl)diisopropylmethylammonium bromide xanthene-9-carboxylate bromide; 2-Hydroxyethyl]diisopropylmethyl-ammonium bromide xanthene-9-carboxylate; 2-Propanaminium, N-methyl-N-(1-methylethyl)-N-(2-((9H-xanthen-9-ylcarbonyl)oxy)ethyl)-, bromide; 2-Propanaminium, N-methyl-N-(1-methylethyl)-N-(2-((9H-xanthen-9-ylcarbonyl)oxy)ethyl)-, bromide (1:1)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antispasmodics
|
||||
| Structure |
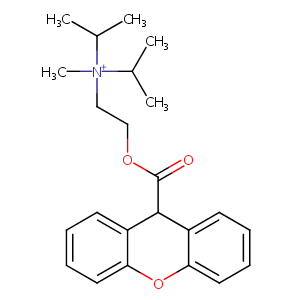
|
Download2D MOL |
|||
| Formula |
C23H30NO3+
|
||||
| InChI |
InChI=1S/C23H30NO3/c1-16(2)24(5,17(3)4)14-15-26-23(25)22-18-10-6-8-12-20(18)27-21-13-9-7-11-19(21)22/h6-13,16-17,22H,14-15H2,1-5H3/q+1
|
||||
| InChIKey |
VVWYOYDLCMFIEM-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 298-50-0
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9709, 5371485, 7764544, 7980411, 8153041, 11111683, 11112101, 11335977, 11361216, 11363764, 11366326, 11368888, 11371864, 11374484, 11377050, 11462188, 11466855, 11467975, 11485075, 11486410, 11489116, 11490698, 11492788, 11494684, 14877274, 29224012, 46507187, 47515349, 47662323, 47736523, 47810790, 47885451, 47959787, 48185034, 48259270, 49698983, 50064654, 50100331, 50103935, 57322535, 68530551, 80339766, 85209927, 90340675, 104307825, 123092451, 124750168, 124881224, 124881225, 124881226
|
||||
| SuperDrug ATC ID |
A03AB05
|
||||
| SuperDrug CAS ID |
cas=000298500
|
||||
| Target and Pathway | |||||
| Target(s) | Muscarinic acetylcholine receptor M1 | Target Info | Antagonist | [536001] | |
| References | |||||
| Ref 538344 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 080927. | ||||
| Ref 540253 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 329). | ||||
| Ref 551871 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

