Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0W9GA
|
||||
| Former ID |
DIB008005
|
||||
| Drug Name |
Levormeloxifene non-steroidal
|
||||
| Synonyms |
Centchroman; Centron; Levormeloxifene; Ormeloxifene; Saheli; NNC-46-0020; Non-steroidal (oral, contraception/cancer); Non-steroidal (oral, contraception/cancer), Central Drug Research Institute (CDRI)
|
||||
| Indication | Breast cancer [ICD9: 174, 175; ICD10:C50] | Approved | [546694] | ||
| Company |
Novo Nordisk A/S; Central Drug Research Institute
|
||||
| Structure |
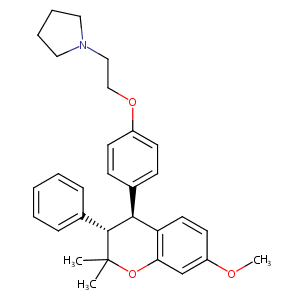
|
Download2D MOL |
|||
| Canonical SMILES |
C1(Oc2c([C@@H]([C@H]1c1ccccc1)c1ccc(cc1)OCCN1CCCC1)ccc(<br />c2)OC)(C)C
|
||||
| Target and Pathway | |||||
| Target(s) | Estrogen receptor | Target Info | Modulator | [526082], [551871] | |
| Pathway Interaction Database | Regulation of nuclear SMAD2/3 signaling | ||||
| Signaling events mediated by HDAC Class II | |||||
| Plasma membrane estrogen receptor signaling | |||||
| LKB1 signaling events | |||||
| Regulation of Telomerase | |||||
| ATF-2 transcription factor network | |||||
| AP-1 transcription factor network | |||||
| FOXM1 transcription factor network | |||||
| Validated nuclear estrogen receptor alpha network | |||||
| Signaling mediated by p38-alpha and p38-beta | |||||
| FOXA1 transcription factor network | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

