Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0WK0P
|
||||
| Former ID |
DIB009011
|
||||
| Drug Name |
Topiroxostat
|
||||
| Synonyms |
FYX-051; Xanthine oxidoreductase inhibitor (oral, gout), Fuji Yakuhin
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Gout [ICD9: 274.00274.1274.8274.9; ICD10:M10] | Phase 2 | [525044] | ||
| Company |
Fuji Yakuhin Kogyo Co Ltd
|
||||
| Structure |
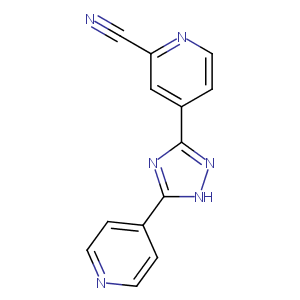
|
Download2D MOL |
|||
| Formula |
C13H8N6
|
||||
| Canonical SMILES |
c1(cc(ccn1)c1n[nH]c(n1)c1ccncc1)C#N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Xanthine dehydrogenase/oxidase | Target Info | Inhibitor | [532545] | |
| PANTHER Pathway | Adenine and hypoxanthine salvage pathway | ||||
| Purine metabolism | |||||
| PathWhiz Pathway | Caffeine Metabolism | ||||
| Purine Metabolism | |||||
| Reactome | Purine catabolism | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

