Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0X3TI
|
||||
| Former ID |
DNCL002922
|
||||
| Drug Name |
SRT3025
|
||||
| Indication | Type 2 diabetes [ICD9: 250; ICD10:E11] | Phase 1 | [523447] | ||
| Company |
Sirtris
|
||||
| Structure |
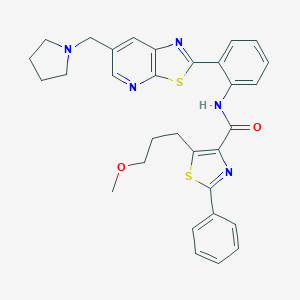
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | NAD-dependent deacetylase sirtuin-1 | Target Info | Modulator | [533296] | |
| PANTHER Pathway | p53 pathway | ||||
| Pathway Interaction Database | p73 transcription factor network | ||||
| Signaling events mediated by HDAC Class III | |||||
| E2F transcription factor network | |||||
| HIF-2-alpha transcription factor network | |||||
| Signaling events mediated by HDAC Class I | |||||
| FoxO family signaling | |||||
| Regulation of Androgen receptor activity | |||||
| Regulation of retinoblastoma protein | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

