Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Y0OA
|
||||
| Former ID |
DNC006449
|
||||
| Drug Name |
OCHNAFLAVONE
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [528052] | ||
| Structure |
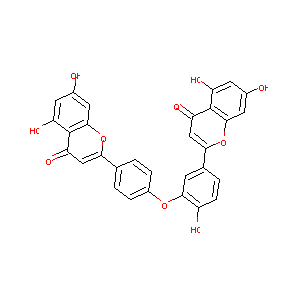
|
Download2D MOL |
|||
| Formula |
C30H18O10
|
||||
| Canonical SMILES |
C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)OC4=C(C=CC(=C4<br />)C5=CC(=O)C6=C(C=C(C=C6O5)O)O)O
|
||||
| InChI |
1S/C30H18O10/c31-16-8-20(34)29-22(36)12-24(39-27(29)10-16)14-1-4-18(5-2-14)38-26-7-15(3-6-19(26)33)25-13-23(37)30-21(35)9-17(32)11-28(30)40-25/h1-13,31-35H
|
||||
| InChIKey |
NNPGECDACGBKDH-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Phospholipase A2, membrane associated | Target Info | Inhibitor | [528052] | |
| BioCyc Pathway | Phospholipases | ||||
| Pathway Interaction Database | Glypican 1 network | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

