Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Y0SY
|
||||
| Former ID |
DIB005001
|
||||
| Drug Name |
E-3620
|
||||
| Indication | Gastric motility disorder [ICD10:K22.4] | Discontinued in Phase 2 | [545633] | ||
| Company |
Eisai Co Ltd
|
||||
| Structure |
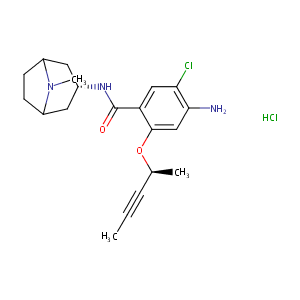
|
Download2D MOL |
|||
| Canonical SMILES |
c1(C(=O)N[C@@H]2CC3N(C(C2)CC3)C)c(cc(c(c1)Cl)N)O[C@H](C<br />#CC)C.Cl
|
||||
| CAS Number |
CAS 151213-85-3
|
||||
| Target and Pathway | |||||
| Target(s) | 5-hydroxytryptamine 4 receptor | Target Info | Modulator | [1572591] | |
| 5-HT 3 receptor | Target Info | Modulator | [1572591] | ||
| PathWhiz Pathway | Excitatory Neural Signalling Through 5-HTR 4 and Serotonin | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

