Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Y7JU
|
||||
| Former ID |
DCL000388
|
||||
| Drug Name |
GW685698X
|
||||
| Synonyms |
Allermist; Avamys; Veramyst; Fluticasone furoate; GW 685698X; GW6; GW-685698; GW-685698X; Veramyst (TN); Fluticasone furoate (JAN/USAN/INN); (6alpha,11beta,16alpha,17alpha)-6,9-Difluoro-17-(((fluoromethyl)thio)carbonyl)-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl-2-furancarboxylate; 6alpha,9-Difluoro-17-(((fluoromethyl)sulfanyl)carbonyl)-11beta-hydroxy-16alpha-methyl-3-oxoandrosta-1,4-dien-17alpha-yl furan-2-carboxylate
|
||||
| Drug Type |
Small molecular drug
|
||||
| Company |
GSK
|
||||
| Structure |
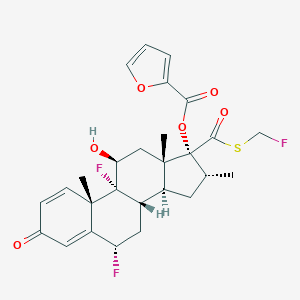
|
Download2D MOL |
|||
| Formula |
C27H29F3O6S
|
||||
| Canonical SMILES |
CC1CC2C3CC(C4=CC(=O)C=CC4(C3(C(CC2(C1(C(=O)SCF)OC(=O)C5<br />=CC=CO5)C)O)F)C)F
|
||||
| InChI |
1S/C27H29F3O6S/c1-14-9-16-17-11-19(29)18-10-15(31)6-7-24(18,2)26(17,30)21(32)12-25(16,3)27(14,23(34)37-13-28)36-22(33)20-5-4-8-35-20/h4-8,10,14,16-17,19,21,32H,9,11-13H2,1-3H3/t14-,16+,17?,19+,21+,24+,25+,26+,27+/m1/s1
|
||||
| InChIKey |
XTULMSXFIHGYFS-KOMYIWBZSA-N
|
||||
| CAS Number |
CAS 397864-44-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
D07AC17; R01AD12; R03BA05
|
||||
| Target and Pathway | |||||
| Target(s) | Glucocorticoid receptor | Target Info | Agonist | [550963] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| NetPath Pathway | IL2 Signaling Pathway | ||||
| TCR Signaling Pathway | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

