Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0ZB7K
|
||||
| Former ID |
DAP000427
|
||||
| Drug Name |
Zopiclone
|
||||
| Synonyms |
Amoban; Amovane; Imovance; Imovane; Limovan; Optidorm; Rhovane; Siaten; Sopivan; Ximovan; Zileze; Zimoclone; Zimovane; Zopicalm; Zopicalma; Zopiclodura; Zopiclona; Zopiclonum; Zopitan; Zorclone; AbZ brand of zopiclone; Aliud brand of zopiclone; Alpharma brand of zopiclone; Aventis Pharma brand of zopiclone; Aventis brand of zopiclone; Azupharma brand of zopiclone; Betapharm brand of zopiclone; Ciclum brand of zopiclone; Clonmel brand of zopiclone; Dolorgeit brand of zopiclone; Gerard brand of zopiclone; Hexal brand of zopiclone; Hormosan brand of zopiclone; Italfarmaco brand of zopiclone; Merck dura brand of zopiclone; Neuraxpharm brand of zopiclone; Norton brand of zopiclone; Opus brand of zopiclone; Pinewood brand of zopiclone; Ratiopharm brand of zopiclone; Rhodiapharm brand of zopiclone; Stadapharm brand of zopiclone; TAD brand of zopiclone; Temmler brand of zopiclone; Teva brand of zopiclone; Zopiclon AL; Zopiclon AZU; Zopiclon AbZ; Zopiclon Stada; Zopiclon TAD; Zopiclon beta; Zopiclon von ct; RP 27 267; RP 27267; Z 4900; Z4900_SIGMA; Amoban (TN); Ct-Arzneimittel brand of zopiclone; Imovane (TN); Lunesta (TN); Novo-zopiclone; Nu-Pharm brand of zopiclone; Nu-Zopiclone; RP-27267; Ran-zopiclone; Ratio-Zopiclone; Rhone-Poulenc Rorer brand of zopiclone; Zimovane (TN); Zopi-Puren; Zopiclon-TEVA; Zopiclon-neuraxpharm; Zopiclon-ratiopharm; Zopiclona [INN-Spanish]; Zopiclone (TN); Zopiclonum [INN-Latin]; Zopinox (TN); Zopiclone (JAN/INN); Zopiclone [BAN:INN:JAN]; Zopiclone, Imovane, Zimovane, Lunesta; [6-(5-chloropyridin-2-yl)-5-oxo-7H-pyrrolo[3,4-b]pyrazin-7-yl] 4-methylpiperazine-1-carboxylate; (+-)-Zopiclone; 1-Piperazinecarboxylic acid, 4-methyl-, 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo(3,4-b)pyrazin-5-yl ester; 1-Piperazinecarboxylic acid, 4-methyl-, 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl ester; 4-Methyl-1-piperazinecarboxylic acid 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo[3,4-b]pyrazin-5-yl ester; 4-Methyl-1-piperazinecarboxylic acid ester with 6-(5-chloro-2-pyridyl)-6,7-dihydro-7-hydroxy-5H-pyrrolo(3,4-b)pyrazin-5-one; 6-(5-Chloro-2-pyridinyl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-yl 4-methyl-1-piperazinecarboxylate; 6-(5-chloro-2-pyridyl)-6,7-dihydro-7-oxo-5H-pyrrolo(3,4-b)pyrazin-5-yl 4-methyl-1-piperazinecarboxylate;6-(5-chloropyrid-2-yl)-5-(4-methylpiperazin-1-yl)carbonyloxy-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazine; 6-(5-chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazin-5-yl 4-methylpiperazine-1-carboxylate
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||
| Company |
Sanofi-Aventis
|
||||
| Structure |
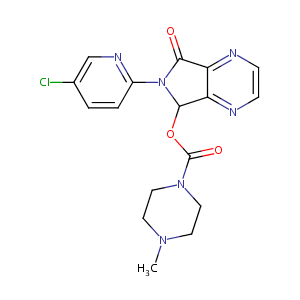
|
Download2D MOL |
|||
| Formula |
C17H17ClN6O3
|
||||
| InChI |
InChI=1S/C17H17ClN6O3/c1-22-6-8-23(9-7-22)17(26)27-16-14-13(19-4-5-20-14)15(25)24(16)12-3-2-11(18)10-21-12/h2-5,10,16H,6-9H2,1H3
|
||||
| InChIKey |
GBBSUAFBMRNDJC-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 43200-80-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
855666, 866104, 5375172, 7848435, 7849623, 7980919, 8153516, 10522775, 12013157, 14780617, 17405824, 24278148, 26747152, 26752065, 29224772, 46505233, 47656611, 47805060, 48403964, 50011925, 50085854, 50085966, 53778376, 56313660, 57309681, 75503738, 81093136, 85788133, 87246425, 92303207, 99437272, 103372219, 103950786, 104310106, 117795243, 124750371, 124757255, 124881860, 124881861, 124881862, 125164059, 125307796, 125325469, 125340634, 125350552, 126666542, 131803007, 134337723, 135001647, 135697518
|
||||
| SuperDrug ATC ID |
N05CF01
|
||||
| SuperDrug CAS ID |
cas=043200802
|
||||
| Target and Pathway | |||||
| Target(s) | Glutamate receptor AMPA subtype | Target Info | Binder | [535660], [535995], [537160] | |
| References | |||||
| Ref 538566 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021476. | ||||
| Ref 542453 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7430). | ||||
| Ref 535660 | Knockouts model the 100 best-selling drugs--will they model the next 100? Nat Rev Drug Discov. 2003 Jan;2(1):38-51. | ||||
| Ref 535995 | The pharmacology and mechanisms of action of new generation, non-benzodiazepine hypnotic agents. CNS Drugs. 2004;18 Suppl 1:9-15; discussion 41, 43-5. | ||||
| Ref 537160 | Eszopiclone: its use in the treatment of insomnia. Neuropsychiatr Dis Treat. 2007 Aug;3(4):441-453. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

