Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08SOF
|
||||
| Former ID |
DAP000102
|
||||
| Drug Name |
Terfenadine
|
||||
| Synonyms |
Aldaban; Allerplus; Cyater; Hisfedin; Rapidal; Seldane; Teldane; Teldanex; Terdin; Terfedura; Terfemundin; Terfen; Terfenadina; Terfenadinum; Terfenidine; Terfex; Ternadin; Triludan; Aliud Brand of Terfenadine; Balkis Saft Spezial; Bial Brand of Terfenadine; Cantabria Brand of Terfenadine; Ct Arzneimittel Brand of Terfenadine; Dolorgiet Brand of Terfenadine; Heumann Brand of Terfenadine; Hoechst Brand of Terfenadine; Merck dura Brand of Terfenadine; Mundipharma Brand of Terfenadine; Ratiopharm Brand of Terfenadine; Sigma Tau Brand of Terfenadine; Stadapharm Brand of Terfenadine; Terfenadin AL; Terfenadin Heumann; Terfenadin Stada; Terfenadin von ct; Wolff Brand of Terfenadine; MDL 9918; RMI 9918; RMI9918; T 9652; Ct-Arzneimittel Brand of Terfenadine; MDL-9918; RMI-9918; Seldane (TN); Sigma-Tau Brand of Terfenadine; Teldane (TN); Terfenadin-ratiopharm; Terfenadina [INN-Spanish]; Terfenadinum [INN-Latin]; Triludan (TN); Terfenadine (JAN/USAN/INN); Terfenadine [USAN:BAN:INN:JAN]; Alpha-(4-[1,1-Dimethylethyl]phenyl)-4-[hydroxydiphenylmethyl]-1-piperidinebutanol; Alpha-(p-tert-Butylphenyl)-4-(hydroxydiphenylmethyl)-1-piperidinebutanol; Alpha-[4-(1,1-Dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1-piperidinebutanol; Alpha-(4-(1,1-Dimethylethyl)phenyl)-4-(hydroxydiphenylmethyl)-1-piperdinebutanol; Alpha-(4-(1,1-Dimethylethyl)phenyl)-4-(hydroxydiphenylmethyl)-1-piperidinebutanol; Alpha-(4-(1,1-dimethylethyl)phenyl)-4-(hydroxydiphenyl-methyl)-1-piperidine butanol; 1-(4-tert-Butylphenyl)-4-(4-(alpha-hydroxybenzhydryl)piperidino)-butan-1-ol; 1-(4-tert-butylphenyl)-4-[4-(hydroxydiphenylmethyl)piperidin-1-yl]butan-1-ol; 1-(4-tert-butylphenyl)-4-[4-[hydroxy(diphenyl)methyl]piperidin-1-yl]butan-1-ol; 1-(p-tert-Butylphenyl)-4-(4'-(alpha-hydroxydiphenylmethyl)-1'-piperidyl)butanol; 1-[4-(1,1-dimethylethyl)phenyl]-4-{4-[hydroxy(diphenyl)methyl]piperidin-1-yl}butan-1-ol
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antihistamines
|
||||
| Company |
Marion Merrell. Dow, Inc.
|
||||
| Structure |
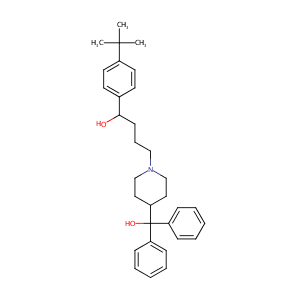
|
Download2D MOL |
|||
| Formula |
C32H41NO2
|
||||
| InChI |
InChI=1S/C32H41NO2/c1-31(2,3)26-18-16-25(17-19-26)30(34)15-10-22-33-23-20-29(21-24-33)32(35,27-11-6-4-7-12-27)28-13-8-5-9-14-28/h4-9,11-14,16-19,29-30,34-35H,10,15,20-24H2,1-3H3
|
||||
| InChIKey |
GUGOEEXESWIERI-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 50679-08-8
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9666, 511394, 855647, 3154381, 5639782, 7847587, 7980761, 8150039, 8153324, 10321465, 10524514, 11336186, 11361425, 11427139, 11462397, 11466166, 11467286, 11485891, 12013836, 14907543, 17405767, 24277779, 26752308, 29224457, 46507007, 47662382, 47662383, 47736580, 47810839, 47810840, 48035221, 48110535, 48416601, 49698808, 49703451, 50015354, 50105228, 50105229, 53778319, 53787217, 56422373, 57322760, 79821290, 85089724, 85209594, 85231279, 85787897, 90341346, 92125182, 92303330
|
||||
| SuperDrug ATC ID |
R06AX12
|
||||
| SuperDrug CAS ID |
cas=050679088
|
||||
| Target and Pathway | |||||
| Target(s) | Histamine H1 receptor | Target Info | Antagonist | [536727], [538157] | |
| PANTHER Pathway | Histamine H1 receptor mediated signaling pathway | ||||
| References | |||||
| Ref 534869 | Comparative tolerability of second generation antihistamines. Drug Saf. 1999 May;20(5):385-401. | ||||
| Ref 539685 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2608). | ||||
| Ref 536727 | Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008 Aug;295(2):C499-513. Epub 2008 May 28. | ||||
| Ref 538157 | Second-generation antihistamines: a comparative review. Drugs. 1999 Jan;57(1):31-47. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

