Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0N9VZ
|
||||
| Former ID |
DND000038
|
||||
| Drug Name |
ADL-5747
|
||||
| Indication | Pain [ICD9: 338, 356.0, 356.8,780; ICD10:G64, G90.0, R52, G89] | Phase 2 | [522936] | ||
| Company |
Pfizer
|
||||
| Structure |
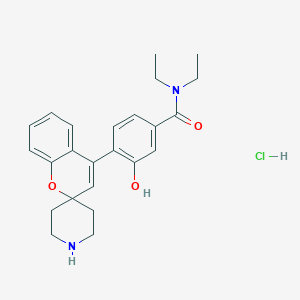
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Delta-type opioid receptor | Target Info | Agonist | [543762] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

