Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0O1HL
|
||||
| Former ID |
DIB011930
|
||||
| Drug Name |
SC-49483
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Acquired immune deficiency syndrome [ICD9: 42; ICD10:B20] | Phase 2 | [521416] | ||
| Company |
GD Searle & Co
|
||||
| Structure |
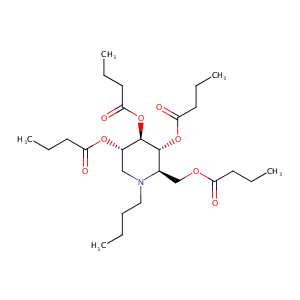
|
Download2D MOL |
|||
| Formula |
C26H45NO8
|
||||
| Canonical SMILES |
[C@@H]1([C@@H]([C@H](CN([C@@H]1COC(=O)CCC)CCCC)OC(=O)CC<br />C)OC(=O)CCC)OC(=O)CCC
|
||||
| CAS Number |
CAS 131262-82-3
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Maltase-glucoamylase, intestinal | Target Info | Inhibitor | [534275] | |
| PathWhiz Pathway | Starch and Sucrose Metabolism | ||||
| WikiPathways | Metabolism of carbohydrates | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

