COVID-19 Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06VSD
|
|||
| Drug Name |
Captopril
|
|||
| Synonyms |
captopril; 62571-86-2; Capoten; L-Captopril; Lopirin; Captopryl; Cesplon; Captolane; Tensoprel; Dilabar; Acepress; Captoril; Tenosbon; Hypertil; Garranil; Alopresin; Lopril; Acepril; Captoprilum; Aceplus; Acediur; Tensobon; Isopresol;Apopril; Asisten; Captoprilum [INN-Latin]; SQ 14225; SQ-14225; SQ 14,225; SA 333; Captopril
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coronavirus Disease 2019 (COVID-19) | Phase 3 | [1] | |
| Other Indication | Hypertension | Approved | [2] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
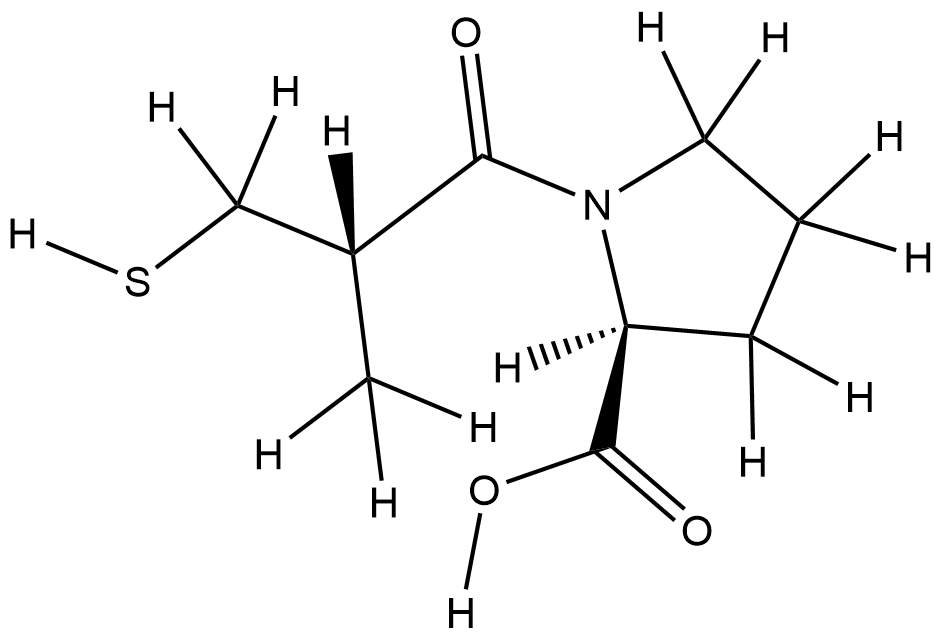 |
Download2D MOL |
||
| Formula |
C9H15NO3S
|
|||
| Canonical SMILES |
CC(CS)C(=O)N1CCCC1C(=O)O
|
|||
| InChI |
1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1
|
|||
| InChIKey |
FAKRSMQSSFJEIM-RQJHMYQMSA-N
|
|||
| CAS Number |
CAS 62571-86-2
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:3380
|
|||
| Target | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN angiotensin-converting enzyme (ACE) | Target Info | Inhibitor | [3] |
| ACE inhibitor Captopril impairs the ACE/angiotensin II/angiotensin-1 receptor pathway, therefore, impairing the integrity of the ACE2/angiotensin 17/MAS (MAS-related G protein-coupled receptor). A disruption of the ACE2/angiotensin 17/MAS pathway could lead to decreased production of ACE2, decreasing chances of SARS-CoV-2 entering the cell. | ||||
| References | Top | |||
|---|---|---|---|---|
| 1 | ClinicalTrials.gov (NCT04345406) Angiotensin Converting Enzyme Inhibitors in Treatment of Covid 19. U.S. National Institutes of Health. | |||
| 2 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 074532. | |||
| 3 | Outcomes in Patients with COVID-19 Infection Taking ACEI/ARB. Curr Cardiol Rep. 2020 Apr 14;22(5):31. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

