Prodrug Information
| Prodrug General Information | Top | |||||
|---|---|---|---|---|---|---|
| Prodrug ID |
DZ4BX6
|
|||||
| Prodrug Name |
Fosaprepitant
|
|||||
| Synonyms |
Ivemend; UNII-6L8OF9XRDC; Fosaprepitant free acid; 6L8OF9XRDC; CHEBI:64321; L-758298; 172673-20-0 (free acid); L-758,298; L 758298; MK-0517; Fosaprepitant [INN:BAN]; fosaprepitantum; C23H22F7N4O6P; Fosaprepitant (INN); SCHEMBL309491; GTPL7623; CHEMBL1199324; CTK4D4303; ZINC3939013; CF0060; AKOS025149465; ACN-040053; AM84605; BCP9000702; CS-1760; DB06717; NCGC00390240-01; AC-25507; HY-14407; Y0425; A11756; D10895; 673F200; Q5473324
Click to Show/Hide
|
|||||
| Indication | Nausea and vomiting [ICD-11: MD90; ICD-10: R11] | Approved | [1] | |||
| Activation |
Prodrug 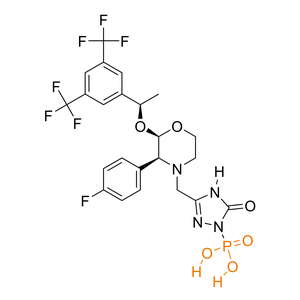
|

|
Parent Drug 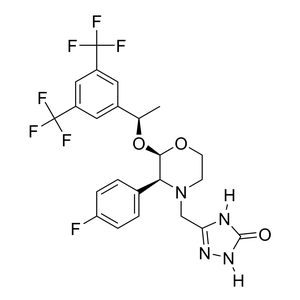
|
|||
| 2D MOL 3D MOL | 2D MOL 3D MOL | |||||
|
(1) Bioconversion Enzyme:
Phosphatase
(EC 3.1)
|
[2] | |||||
| Prodrug Strategy |
Classical prodrug strategy
[Carrier linked prodrug]
|
|||||
| Improved property |
Increase solubility
|
[3] | ||||
| Formula |
C23H22F7N4O6P
|
|||||
| Canonical SMILES |
C[C@H](C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F)O[C@@H]2[C@@H](N(CCO2)CC3=NN(C(=O)N3)P(=O)(O)O)C4=CC=C(C=C4)F
|
|||||
| InChI |
1S/C23H22F7N4O6P/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)40-20-19(13-2-4-17(24)5-3-13)33(6-7-39-20)11-18-31-21(35)34(32-18)41(36,37)38/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H,31,32,35)(H2,36,37,38)/t12-,19+,20-/m1/s1
|
|||||
| InChIKey |
BARDROPHSZEBKC-OITMNORJSA-N
|
|||||
| CAS Number |
CAS 172673-20-0
|
|||||
| PubChem Compound ID | ||||||
| ChEBI ID |
CHEBI:64321
|
|||||
| Parent Drug General Information | Top | |||||
|---|---|---|---|---|---|---|
| Parent Drug ID |
DH86JL
|
|||||
| Parent Drug Name |
Aprepitant
|
|||||
| Synonyms |
MK-0869; MK-869; L-754030; CHEMBL1471; (1R,2R,3R)-Aprepitant; CHEMBL1471; Cinvanti; Emend; L-754,030; L982; MK 0869; MK 869; MK-0869; MK-869; MK0869; ONO 7436; ONO-7436; Aprepitantum
Click to Show/Hide
|
|||||
| Formula |
C23H21F7N4O3
|
|||||
| Canonical SMILES |
C[C@H](C1=CC(=CC(=C1)C(F)(F)F)C(F)(F)F)O[C@@H]2[C@@H](N(CCO2)CC3=NNC(=O)N3)C4=CC=C(C=C4)F
|
|||||
| InChI |
1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1
|
|||||
| InChIKey |
ATALOFNDEOCMKK-OITMNORJSA-N
|
|||||
| CAS Number |
CAS 170729-80-3
|
|||||
| PubChem Compound ID | ||||||
| ChEBI ID |
CHEBI:499361
|
|||||
| Target and Pathway | Top | |||||
|---|---|---|---|---|---|---|
| Target(s) | Substance-P receptor (TACR1) | Target Info | Antagonist | [1] | ||
| KEGG Pathway | Calcium signaling pathway | |||||
| Neuroactive ligand-receptor interaction | ||||||
| Measles | ||||||
| Panther Pathway | CCKR signaling map ST | |||||
| Reactome | G alpha (q) signalling events | |||||
| WikiPathways | SIDS Susceptibility Pathways | |||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||||
| Spinal Cord Injury | ||||||
| Peptide GPCRs | ||||||
| GPCR ligand binding | ||||||
| GPCR downstream signaling | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2018 | |||||
| REF 2 | Fosaprepitant and aprepitant: an update of the evidence for their place in the prevention of chemotherapy-induced nausea and vomiting. Core Evid. 2010 Oct 21;5:77-90. | |||||
| REF 3 | Clinical outcomes of theophylline use as add-on therapy in patients with chronic obstructive pulmonary disease: A propensity score matching analysis. Chron Respir Dis. Jan-Dec 2019;16:1479973118815694. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

