Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08ROP
|
||||
| Former ID |
DAP000636
|
||||
| Drug Name |
Leflunomide
|
||||
| Synonyms |
Arava; Leflunomid; Leflunomida; Leflunomidum; Lefunamide; Aventis Behring Brand of Leflunomide; Aventis Brand of Leflunomide; Aventis Pharma Brand of Leflunomide; Hoechst Brand of Leflunomide; HWA 486; L 5025; SU 101; SU101; Arava (TN); Arava, Leflunomide; HWA-486; Leflunomida [INN-Spanish]; Leflunomide [USAN:INN]; Leflunomidum [INN-Latin]; Lefunomide [Inn-Spanish]; RS-34821; SU 101 (pharmaceutical); SU-101; AP-501/42475599; Leflunomide (JAN/USAN/INN); N-(4-trifluoromethyphenyl)-5-methylisoxazole-4-carboxamide; N-(4'-Trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide; Alpha,alpha,alpha-Trifluoro-5-methyl-4-isoxazolecarboxy-p-toluidide; 4-Isoxazolecarboxamide, 5-methyl-N-(4-(trifluoromethyl)phenyl; 4-isoxazolecarboxamide,5-methyl-N-(4-(trifluoromethyl)phenyl); 5-Methyl-N-(4-(trifluoromethyl)phenyl)-4-isoxazolecarboxamide; 5-Methylisoxazole-4-(4-trifluoromethyl)carboxanilide; 5-Methylisoxazole-4-(4-trifluoromethylcarboxanilide); 5-Methylisoxazole-4-carboxylic acid (4-trifluoromethyl)anilide; 5-methyl-N-[4-(trifluoromethyl)phenyl]-1,2-oxazole-4-carboxamide; 5-methyl-N-[4-(trifluoromethyl)phenyl]-4-isoxazolecarboxamide; 5-methyl-N-[4-(trifluoromethyl)phenyl]isoxazole-4-carboxamide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiinflammatory Agents
|
||||
| Company |
Sanofi-Aventis
|
||||
| Structure |
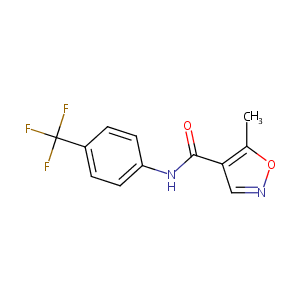
|
Download2D MOL |
|||
| Formula |
C12H9F3N2O2
|
||||
| InChI |
InChI=1S/C12H9F3N2O2/c1-7-10(6-16-19-7)11(18)17-9-4-2-8(3-5-9)12(13,14)15/h2-6H,1H3,(H,17,18)
|
||||
| InChIKey |
VHOGYURTWQBHIL-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 75706-12-6
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
10107, 517091, 604245, 855764, 866530, 6899003, 7847814, 7979735, 8150074, 8152456, 10321188, 11111382, 11111383, 11336091, 11361330, 11363216, 11365778, 11368340, 11376502, 11462302, 11466800, 11467920, 11486519, 11494136, 11528672, 11533365, 12013774, 15221872, 17405210, 24278516, 26612550, 26746985, 26746986, 26746987, 29223013, 46506013, 47589061, 47662351, 47736556, 47885478, 48110514, 48259307, 48259308, 48416160, 49698814, 49835013, 50100264, 50104054, 50104055, 50104056
|
||||
| ChEBI ID |
ChEBI:6402
|
||||
| SuperDrug ATC ID |
L04AA13
|
||||
| SuperDrug CAS ID |
cas=075706126
|
||||
| Target and Pathway | |||||
| Target(s) | Dihydroorotate dehydrogenase, mitochondrial | Target Info | Inhibitor | [534936], [537837], [538007], [538113] | |
| KEGG Pathway | Pyrimidine metabolism | ||||
| Metabolic pathways | |||||
| PathWhiz Pathway | Pyrimidine Metabolism | ||||
| Reactome | Pyrimidine biosynthesis | ||||
| WikiPathways | Metabolism of nucleotides | ||||
| References | |||||
| Ref 532210 | Nat Rev Drug Discov. 2013 Feb;12(2):87-90. | ||||
| Ref 536772 | New drugs in development for the treatment of endometriosis. Expert Opin Investig Drugs. 2008 Aug;17(8):1187-202. | ||||
| Ref 541907 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6825). | ||||
| Ref 534936 | Identification and characterization of potential new therapeutic targets in inflammatory and autoimmune diseases. Eur J Biochem. 1999 Dec;266(3):1184-91. | ||||
| Ref 537837 | Inhibition of dihydroorotate dehydrogenase by the immunosuppressive agent leflunomide. Biochem Pharmacol. 1995 Sep 7;50(6):861-7. | ||||
| Ref 538007 | Dihydroorotate dehydrogenase is a target for the biological effects of leflunomide. Transplant Proc. 1996 Dec;28(6):3088-91. | ||||
| Ref 538113 | Expression, purification, and characterization of histidine-tagged rat and human flavoenzyme dihydroorotate dehydrogenase. Protein Expr Purif. 1998 Aug;13(3):414-22. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

