Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Y7YD
|
||||
| Former ID |
DIB006364
|
||||
| Drug Name |
SMT-C1100
|
||||
| Synonyms |
BMN-195; SMT-C1100; Utrophin modulators (Duchenne muscular dystrophy); VOXC-1100; Utrophin modulators (Duchennemuscular dystrophy), VASTox; Utorphin upregulators (Duchenne muscular dystrophy), VASTox/University of Oxford; Utrophin modulators (Duchenne muscular dystrophy), Summit/BioMarin; Utropine inducer (Duchenne muscular dystrophy), Summit/BioMarin
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Duchenne muscular dystrophy [ICD10:G71.0] | Phase 1 | [525109] | ||
| Company |
Summit Corp plc
|
||||
| Structure |
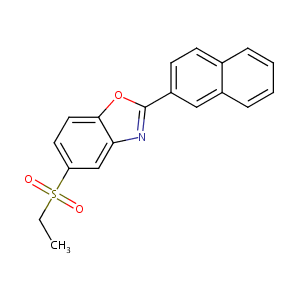
|
Download2D MOL |
|||
| Formula |
C19H15NO3S
|
||||
| Canonical SMILES |
O=S(=O)(c1ccc2oc(nc2c1)c1ccc2c(c1)cccc2)CC
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Utrophin | Target Info | Regulator (upregulator) | [533130] | |
| WikiPathways | Primary Focal Segmental Glomerulosclerosis FSGS | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

