Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Z5AV
|
||||
| Former ID |
DNCL003519
|
||||
| Drug Name |
E5501
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Idiopathic thrombocytopenic purpura [ICD9: 287.31; ICD10:D69.3] | Phase 3 | [523622] | ||
| Company |
Eisai
|
||||
| Structure |
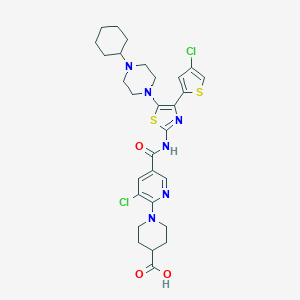
|
Download2D MOL |
|||
| Formula |
C33H38Cl2N6O7S2
|
||||
| Canonical SMILES |
C1CCC(CC1)N2CCN(CC2)C3=C(N=C(S3)NC(=O)C4=CC(=C(N=C4)N5C<br />CC(CC5)C(=O)O)Cl)C6=CC(=CS6)Cl.C(=CC(=O)O)C(=O)O
|
||||
| InChI |
1S/C29H34Cl2N6O3S2.C4H4O4/c30-20-15-23(41-17-20)24-27(37-12-10-35(11-13-37)21-4-2-1-3-5-21)42-29(33-24)34-26(38)19-14-22(31)25(32-16-19)36-8-6-18(7-9-36)28(39)40;5-3(6)1-2-4(7)8/h14-18,21H,1-13H2,(H,39,40)(H,33,34,38);1-2H,(H,5,6)(H,7,8)/b;2-1-
|
||||
| InChIKey |
MISPBGHDNZYFNM-BTJKTKAUSA-N
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| Target and Pathway | |||||
| Target(s) | Thrombopoietin Receptor | Target Info | Agonist | [543468] | |
| WikiPathways | Platelet Aggregation (Plug Formation) | ||||
| References | |||||
| Ref 523622 | ClinicalTrials.gov (NCT01433978) A Phase 3, Multicenter, Randomized, Double-blind, Active-controlled, Parallel-group Trial With an Open-label Extension Phase to Evaluate the Efficacy and Safety of Oral E5501 Versus Eltrombopag, in Adults With Chronic Immune Thrombocytopenia (Idiopathic Thrombocytopenic Purpura). U.S. National Institutes of Health. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

