Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Z9NY
|
||||
| Former ID |
DIB016803
|
||||
| Drug Name |
API-023
|
||||
| Synonyms |
AGN-201904; Proton pump inhibitor prodrug, Alevium; Proton pump inhibitor prodrug, Allergan; AGN-201904-Z; Omeprazole prodrug (GERD), Alevium
|
||||
| Indication | Gastroesophageal reflux disease [ICD9: 140-229, 530; ICD10:K21] | Phase 1 | [521830] | ||
| Company |
Allergan Inc
|
||||
| Structure |
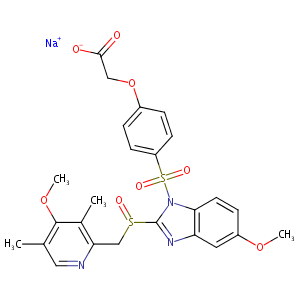
|
Download2D MOL |
|||
| Canonical SMILES |
c1(nc2c(n1S(=O)(=O)c1ccc(cc1)OCC(=O)[O-])ccc(c2)OC)S(=O<br />)Cc1c(c(c(cn1)C)OC)C.[Na+]
|
||||
| CAS Number |
CAS 651728-41-5
|
||||
| Target and Pathway | |||||
| Target(s) | Potassium-transporting ATPase alpha chain 1 | Target Info | Inhibitor | [543965] | |
| PathWhiz Pathway | Gastric Acid Production | ||||
| Reactome | Ion transport by P-type ATPases | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

