Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D01TMQ
|
||||
| Former ID |
DCL000733
|
||||
| Drug Name |
Bupropion+naltrexone
|
||||
| Synonyms |
Contrave (TN)
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Obesity [ICD9: 278; ICD10:E66] | Phase 3 | [532233] | ||
| Company |
Orexigen Therapeutics
|
||||
| Structure |
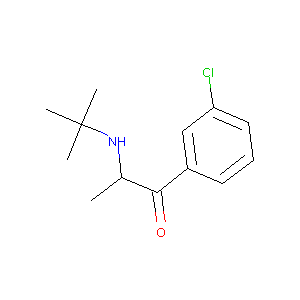
|
Download2D MOL |
|||
| Formula |
C13H18ClNO
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Dopamine reuptake | Target Info | Inhibitor | [536710] | |
| Sodium-dependent noradrenaline transporter | Target Info | Inhibitor | [536710] | ||
| Opioid receptor | Target Info | Antagonist | [536710] | ||
| PANTHER Pathway | Adrenaline and noradrenaline biosynthesis | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

