COVID-19 Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04AGG
|
|||
| Drug Name |
Favipiravir
|
|||
| Synonyms |
AIDS121660; Favipiravir (JAN/INN); 3-bromo-5-hydroxy-4-methyl-2,6-dinitro-benzoic Acid; T705
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coronavirus Disease 2019 (COVID-19) | Phase 3 | [1], [2] | |
| Other Indication | Influenza virus infection | Approved | [3] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
Toyama Chemical
|
|||
| Structure |
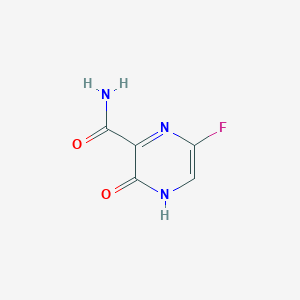 |
Download2D MOL |
||
| Formula |
C5H4FN3O2
|
|||
| Canonical SMILES |
C1=C(N=C(C(=O)N1)C(=O)N)F
|
|||
| InChI |
1S/C5H4FN3O2/c6-2-1-8-5(11)3(9-2)4(7)10/h1H,(H2,7,10)(H,8,11)
|
|||
| InChIKey |
ZCGNOVWYSGBHAU-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 259793-96-9
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:134722
|
|||
| Target | Top | |||
|---|---|---|---|---|
| Target(s) | COVID-19 RNA-directed RNA polymerase (RdRp) | Target Info | Inhibitor | [4], [5] |
| Favipiravir effectively inhibits the RNA-dependent RNA polymerase of RNA viruses and a recent study reported its activity against 2019-nCoV (EC50 = 61.88 microM in Vero E6 cells). | ||||
| References | Top | |||
|---|---|---|---|---|
| 1 | ClinicalTrials.gov (NCT04336904) Clinical Study To Evaluate The Performance And Safety Of Favipiravir in COVID-19 | |||
| 2 | ChiCTR.org.cn (ChiCTR2000030254) the Efficacy and Safety of Favipiravir for novel coronavirusinfected pneumonia: A multicenter, randomized, open, positive, parallel-controlled clinical study | |||
| 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| 4 | Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv. Posted April 15, 2020. | |||
| 5 | Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 2020 Mar;19(3):149-150. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

