COVID-19 Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05ULR
|
|||
| Drug Name |
Sildenafil
|
|||
| Synonyms |
sildenafil; 139755-83-2; VIAGRA; Sildenafil [INN:BAN]; UK-92480; UNII-3M7OB98Y7H; UK-92,480-10; C22H30N6O4S; HSDB 7305; CHEMBL192; UK 92480-10; CHEBI:9139; 3M7OB98Y7H; BNRNXUUZRGQAQC-UHFFFAOYSA-N; Sildenafil
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coronavirus Disease 2019 (COVID-19) | Phase 3 | [1] | |
| Other Indication | Erectile dysfunction | Approved | [2] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
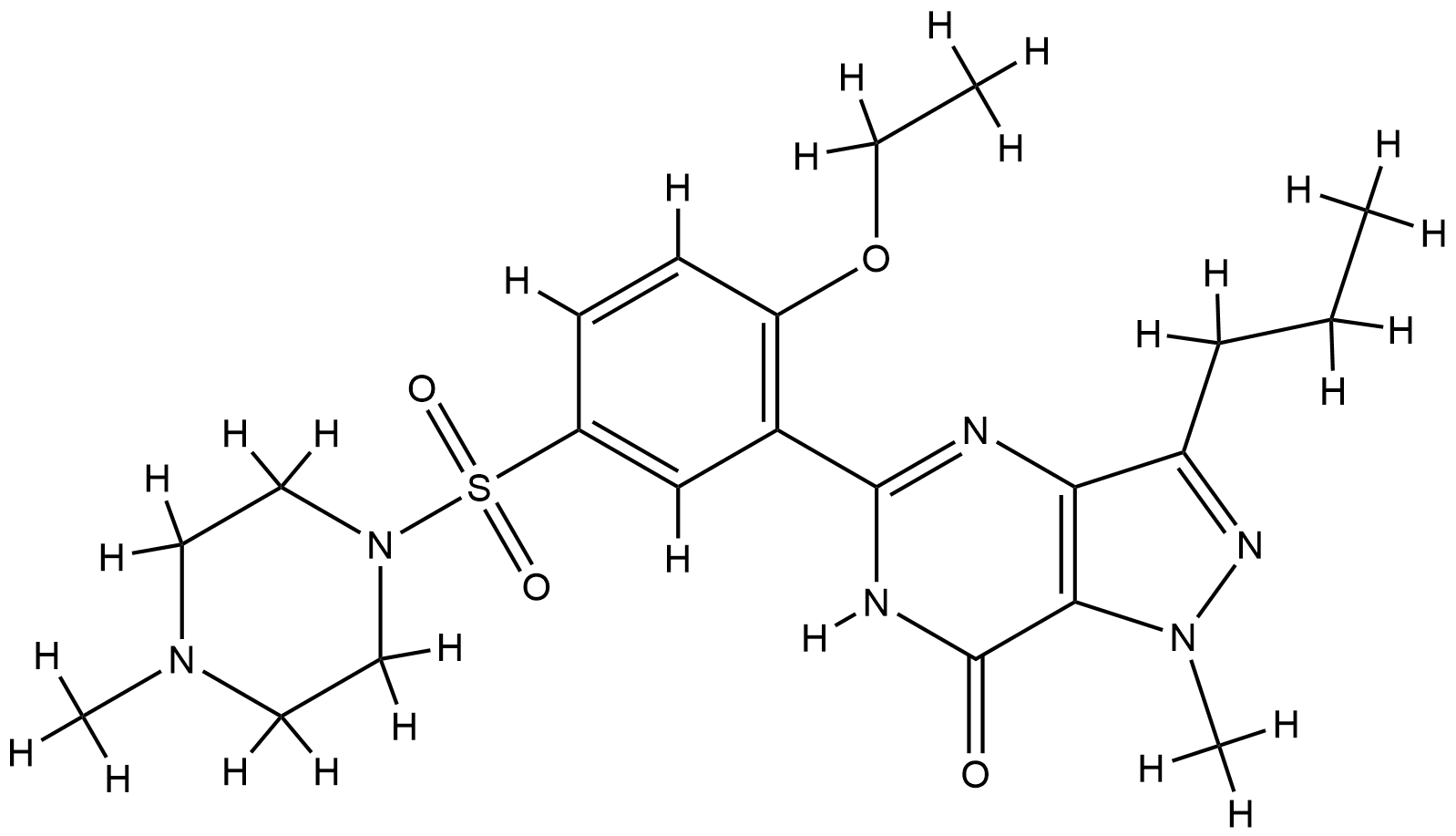 |
Download2D MOL |
||
| Formula |
C22H30N6O4S
|
|||
| Canonical SMILES |
CCCC1=NN(C2=C1N=C(NC2=O)C3=C(C=CC(=C3)S(=O)(=O)N4CCN(CC4)C)OCC)C
|
|||
| InChI |
1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29)
|
|||
| InChIKey |
BNRNXUUZRGQAQC-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 139755-83-2
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:9139
|
|||
| Target | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN phosphodiesterase type 5 (PDE5) | Target Info | Inhibitor | [3], [4] |
| Sildenafil can increase the level of NO through PDE5 inhibiton, which further prevents the replication cycle of SARS CoV/SARS CoV-2. | ||||
| References | Top | |||
|---|---|---|---|---|
| 1 | ClinicalTrials.gov (NCT04304313) A Pilot Study of Sildenafil in COVID-19. U.S. National Institutes of Health. | |||
| 2 | FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 206401. | |||
| 3 | Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol. 2005 Feb;79(3):1966-9. doi: 10.1128/JVI.79.3.1966-1969.2005. | |||
| 4 | Any possible role of phosphodiesterase type 5 inhibitors in the treatment of severe COVID19 infections A lesson from urology. Clin Immunol. 2020 May;214:108414. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

