COVID-19 Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0DG0P
|
|||
| Drug Name |
Triflupromazine
|
|||
| Synonyms |
Adazine; Fluopromazine; Fluorofen; Neoprin; Nivoman; Psyquil; Siquil; Trifluopromazine; Triflupromazina; Triflupromazinum;Vesprin; Vetame; Fluopromazine monohydrochloride; Trifluopromazine hydrochloride; Triflupromazine [INN]; Triflupromazina [INN-Spanish]; Triflupromazinum [INN-Latin]; Vesprin (TN); Triflupromazine (USP/INN)
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Middle East Respiratory Syndrome (MERS) | Investigative | [1] | Severe acute respiratory syndrome (SARS) | Investigative | [1] |
| Other Indication | Schizophrenia | Withdrawn from market | [2] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Company |
Bristol-Myers Squibb
|
|||
| Structure |
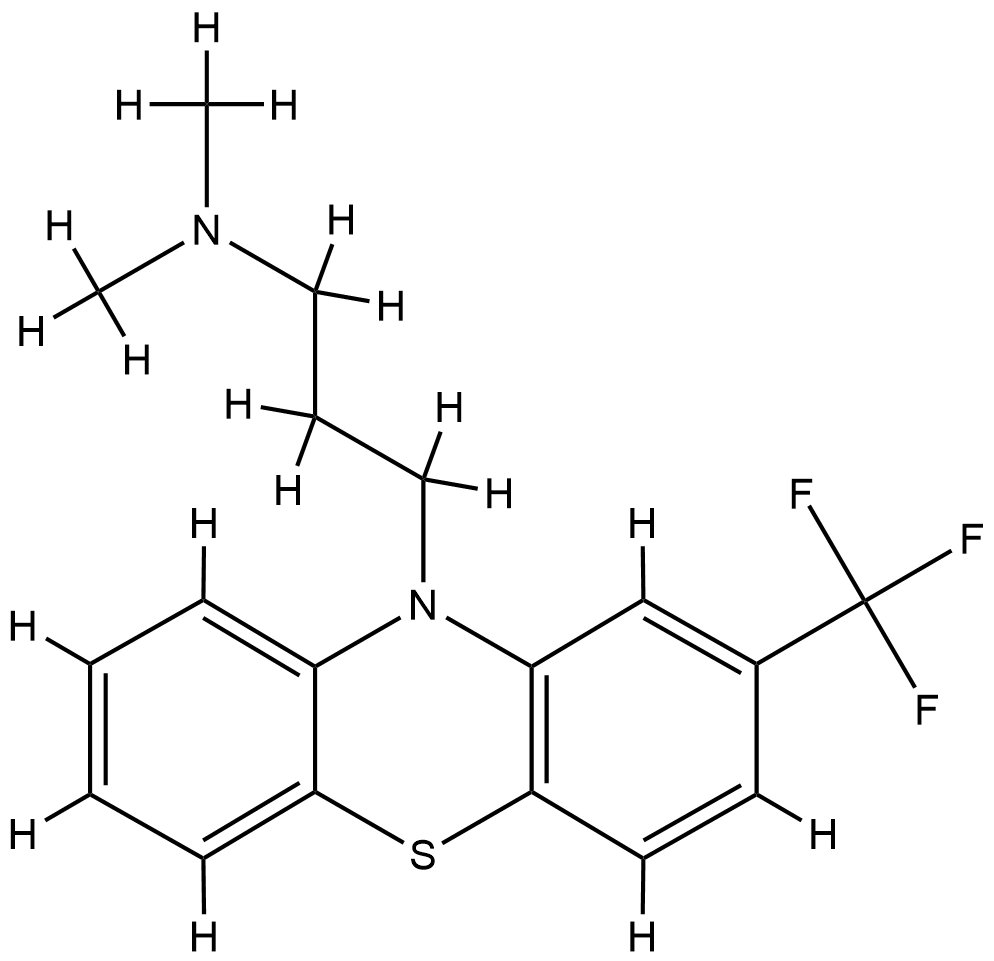 |
Download2D MOL |
||
| Formula |
C18H19F3N2S
|
|||
| Canonical SMILES |
CN(C)CCCN1C2=CC=CC=C2SC3=C1C=C(C=C3)C(F)(F)F
|
|||
| InChI |
1S/C18H19F3N2S/c1-22(2)10-5-11-23-14-6-3-4-7-16(14)24-17-9-8-13(12-15(17)23)18(19,20)21/h3-4,6-9,12H,5,10-11H2,1-2H3
|
|||
| InChIKey |
XSCGXQMFQXDFCW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 146-54-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
5664289, 7847456, 8153424, 10508360, 11111873, 11111874, 11335983, 11361222, 11362929, 11365491, 11368053, 11374201, 11376215, 11405424, 11405821, 11462194, 11466081, 11467201, 11485862, 11492556, 11493909, 14925496, 24263033, 29224607, 46507344, 47365232, 47440302, 47440303, 47662324, 47662325, 47736524, 48035163, 48259273, 48259274, 48334534, 48416663, 49698933, 50006493, 50100355, 50104262, 50839748, 56394865, 57322847, 85209834, 90341005, 103024193, 103179080, 103841663, 103957782, 104309608
|
|||
| ChEBI ID |
CHEBI:9711
|
|||
| Target | Top | |||
|---|---|---|---|---|
| Target(s) | HUMAN clathrin-mediated endocytosis (RME) | Target Info | Inhibitor | [1] |
| Triflupromazine exhibits inhibitory activity against clathrin-mediated endocytosis of SARS-CoV-2. | ||||
| References | Top | |||
|---|---|---|---|---|
| 1 | Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob Agents Chemother. 2014 Aug;58(8):4885-93. | |||
| 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

