COVID-19 Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0J9EI
|
|||
| Drug Name |
Lopinavir + ritonavir
|
|||
| Synonyms |
Aluviran + norvir; Kaletra
|
|||
| Drug Type |
Combination drug
|
|||
| Indication | Coronavirus Disease 2019 (COVID-19) | Phase 3 | [1] | |
| Other Indication | Human immunodeficiency virus infection | Approved | [2] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
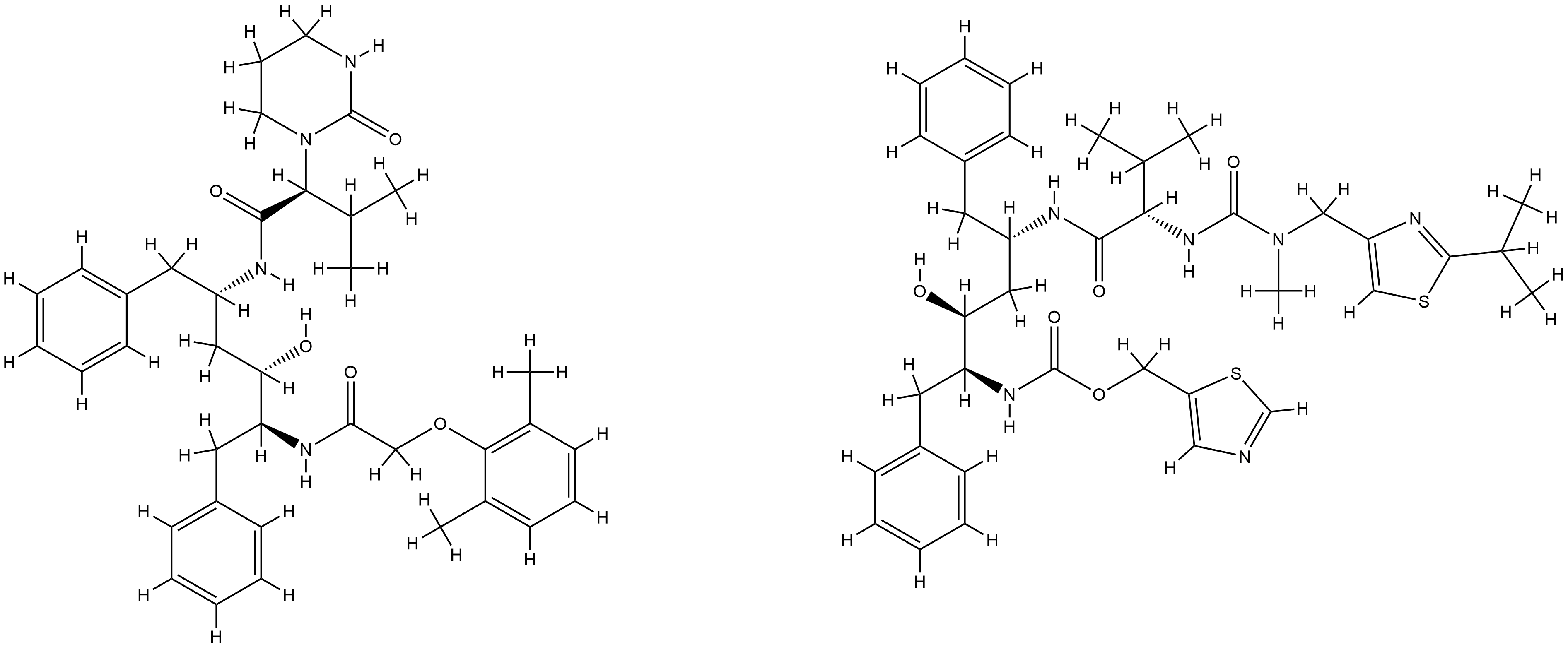 |
Download2D MOL
|
||
| Formula |
C74H96N10O10S2
|
|||
| Canonical SMILES |
CC1=C(C(=CC=C1)C)OCC(=O)NC(CC2=CC=CC=C2)C(CC(CC3=CC=CC=C3)NC(=O)C(C(C)C)N4CCCNC4=O)O.CC(C)C1=NC(=CS1)CN(C)C(=O)NC(C(C)C)C(=O)NC(CC2=CC=CC=C2)CC(C(CC3=CC=CC=C3)NC(=O)OCC4=CN=CS4)O
|
|||
| InChI |
1S/C37H48N6O5S2.C37H48N4O5/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30;1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46);5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t28-,31-,32-,33-;30-,31-,32-,34-/m00/s1
|
|||
| InChIKey |
OFFWOVJBSQMVPI-RMLGOCCBSA-N
|
|||
| CAS Number |
CAS 369372-47-4
|
|||
| PubChem Compound ID | ||||
| Target | Top | |||
|---|---|---|---|---|
| Target(s) | COVID-19 3C-like protease (3CLpro) | Target Info | Inhibitor | [3] |
| Lopinavir/ritonavir blocks viral replication of SARS-CoV-2 via inhibiting the 3C-like protease (3CLpro). | ||||
| References | Top | |||
|---|---|---|---|---|
| 1 | ClinicalTrials.gov (NCT04315948) Trial of Treatments for COVID-19 in Hospitalized Adults | |||
| 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| 3 | Coronavirus puts drug repurposing on the fast track. Nat Biotechnol. 2020 Apr;38(4):379-381. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

