Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D00NPT
|
|||
| Former ID |
DNC000949
|
|||
| Drug Name |
Minodronate
|
|||
| Synonyms |
Minodronic acid; 180064-38-4; (1-Hydroxy-2-(imidazo[1,2-a]pyridin-3-yl)ethane-1,1-diyl)diphosphonic acid; Recalbon; Bonoteo; BPH 261; ONO 5920; YH 529; Phosphonic acid, (1-hydroxy-2-imidazo(1,2-a)pyridin-3-ylethylidene)bis-; UNII-40SGR63TGL; YM 529; 40SGR63TGL; Ono-5920; NSC725590; YH-529; 127657-42-5; NCGC00183829-01; AK119862; Phosphonic acid, (1-hydroxy-2-imidazo[1,2-a]pyridin-3-ylethylidene)bis-; (1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosphonoethyl)phosphonic acid; minodronic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Osteoporosis [ICD-11: FB83.0; ICD-10: M85.8] | Approved | [1], [2] | |
| Structure |
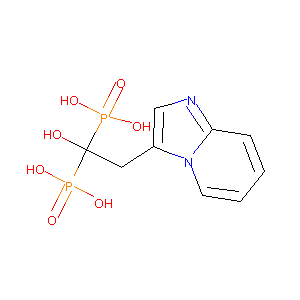 |
Download2D MOL |
||
| Formula |
C9H12N2O7P2
|
|||
| Canonical SMILES |
C1=CC2=NC=C(N2C=C1)CC(O)(P(=O)(O)O)P(=O)(O)O
|
|||
| InChI |
1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18)
|
|||
| InChIKey |
VMMKGHQPQIEGSQ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 180064-38-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7843852, 10242764, 12014461, 14874799, 29196193, 29196330, 29309444, 48426327, 53837734, 56374449, 57343615, 75971565, 87457604, 93309669, 99375738, 103314069, 104374011, 124894544, 125823092, 126651847, 126666053, 129496050, 131302852, 135268217, 135649663, 137084388, 141698785, 144206806, 151995601, 160650211, 160655146, 162205070, 162222716, 163416705, 164777754, 164779842, 164779843, 171578885, 175438067, 179322955, 184547763, 186024269, 196109800, 198979870, 206246374, 223589663, 223662192, 224339905, 226511683, 249828272
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Geranyltranstransferase (FDPS) | Target Info | Inhibitor | [3], [4] |
| BioCyc | Superpathway of geranylgeranyldiphosphate biosynthesis I (via mevalonate) | |||
| Superpathway of cholesterol biosynthesis | ||||
| Trans, trans-farnesyl diphosphate biosynthesis | ||||
| Geranylgeranyldiphosphate biosynthesis | ||||
| KEGG Pathway | Terpenoid backbone biosynthesis | |||
| Metabolic pathways | ||||
| Biosynthesis of antibiotics | ||||
| Influenza A | ||||
| HTLV-I infection | ||||
| NetPath Pathway | TCR Signaling Pathway | |||
| Panther Pathway | Cholesterol biosynthesis | |||
| Pathwhiz Pathway | Steroid Biosynthesis | |||
| Reactome | Cholesterol biosynthesis | |||
| Activation of gene expression by SREBF (SREBP) | ||||
| WikiPathways | Activation of Gene Expression by SREBP (SREBF) | |||
| SREBP signalling | ||||
| Cholesterol Biosynthesis | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007280) | |||
| REF 3 | Synthesis, chiral high performance liquid chromatographic resolution and enantiospecific activity of a potent new geranylgeranyl transferase inhibitor, 2-hydroxy-3-imidazo[1,2-a]pyridin-3-yl-2-phosphonopropionic acid. J Med Chem. 2010 May 13;53(9):3454-64. | |||
| REF 4 | Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001 Feb;296(2):235-42. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

