Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02JNM
|
|||
| Former ID |
DAP001188
|
|||
| Drug Name |
Desonide
|
|||
| Synonyms |
Apolar; DesOwen; Desilux; Desonate; Desonida; Desonidum; Flusemidon; Hamiltoderm; Locapred; Prednacinolone; Prenacid; Reticus; Sterax; Steroderm; Topifug; Tridesilon; Tridesonit; Verdeso; Zotinar; Desfluorotriamcinolone acetonide; D 2083; D-2083; Desonida [INN-Spanish]; Desonidum [INN-Latin]; Desowen (TN); Verdeso (TN);Verdeso Foam (TN); Desonide (USAN/INN); Desonide [USAN:INN:BAN]; Locapred, Topifug, Tridesilon, Desonide; (4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one; 11beta,16alpha,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone; 11beta,21-dihydroxy-16alpha,17-[(1-methylethylidene)bis(oxy)]pregna-1,4-diene-3,20-dione; 11beta,21-dihydroxy-16alpha,17-isopropylidenedioxypregna-1,4-diene-3,20-dione; 11beta,21-dihydroxy-16alpha,17alpha-isopropylidenedioxypregna-1,4-diene-3,20-dione; 16-alpha-Hydroxyprednisole-16,17-acetonide; 16alpha,17alpha-isopropylidenedioxyprednisolone; 16alpha-hydroxyprednisole-16,17-acetonide; 16alpha-hydroxyprednisolone-16alpha,17-acetonide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Atopic dermatitis [ICD-11: EA80; ICD-10: L20; ICD-9: 691.8, 692.9] | Approved | [1], [2] | |
| Therapeutic Class |
Antiinflammatory Agents
|
|||
| Structure |
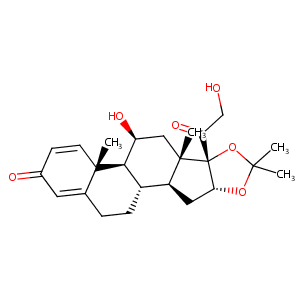 |
Download2D MOL |
||
| Formula |
C24H32O6
|
|||
| Canonical SMILES |
CC1(OC2CC3C4CCC5=CC(=O)C=CC5(C4C(CC3(C2(O1)C(=O)CO)C)O)C)C
|
|||
| InChI |
1S/C24H32O6/c1-21(2)29-19-10-16-15-6-5-13-9-14(26)7-8-22(13,3)20(15)17(27)11-23(16,4)24(19,30-21)18(28)12-25/h7-9,15-17,19-20,25,27H,5-6,10-12H2,1-4H3/t15-,16-,17-,19+,20+,22-,23-,24+/m0/s1
|
|||
| InChIKey |
WBGKWQHBNHJJPZ-LECWWXJVSA-N
|
|||
| CAS Number |
CAS 638-94-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
7979048, 11056247, 14855707, 17397789, 39340794, 46506186, 49834374, 57359343, 71840210, 74651042, 85403370, 103771092, 104179089, 104253319, 124757438, 125164242, 134338433, 134975842, 135653473, 135692232, 136311366, 137125311, 139999928, 144206156, 160964591, 162178659, 170464832, 175268455, 175612227, 176484695, 178103645, 179150829, 210279389, 210281712, 224521289, 226395768, 251915933, 251917281, 252347928
|
|||
| ChEBI ID |
CHEBI:204734
|
|||
| ADReCS Drug ID | BADD_D00619 ; BADD_D02350 | |||
| SuperDrug ATC ID |
D07AB08; S01BA11
|
|||
| SuperDrug CAS ID |
cas=000638948
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Steroid hormone receptor ERR (ESRR) | Target Info | Modulator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7066). | |||
| REF 2 | Emerging drugs for ocular allergy. Expert Opin Emerg Drugs. 2005 Aug;10(3):505-20. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

