Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02VIT
|
|||
| Former ID |
DCL000271
|
|||
| Drug Name |
Arbidol
|
|||
| Synonyms |
Umifenovir; Arbidole; MC-101
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Virus infection [ICD-11: 1A24-1D9Z] | Approved | [1] | |
| Company |
Good Earth Medicine
|
|||
| Structure |
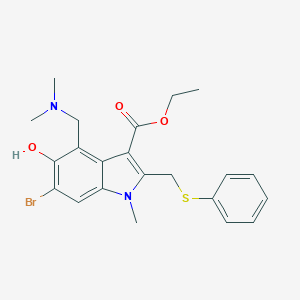 |
Download2D MOL |
||
| Formula |
C22H25BrN2O3S
|
|||
| Canonical SMILES |
CCOC(=O)C1=C(N(C2=CC(=C(C(=C21)CN(C)C)O)Br)C)CSC3=CC=CC=C3
|
|||
| InChI |
1S/C22H25BrN2O3S/c1-5-28-22(27)20-18(13-29-14-9-7-6-8-10-14)25(4)17-11-16(23)21(26)15(19(17)20)12-24(2)3/h6-11,26H,5,12-13H2,1-4H3
|
|||
| InChIKey |
KCFYEAOKVJSACF-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 131707-25-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:134730
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Influenza Hemagglutinin (Influ HA) | Target Info | Inhibitor | [2], [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Pharmacokinetic properties and bioequivalence of two formulations of arbidol: an open-label, single-dose, randomized-sequence, two-period crossover study in healthy Chinese male volunteers. Clin Ther. 2009 Apr;31(4):784-92. | |||
| REF 3 | Investigation of triazavirin antiviral activity against influenza A virus (H5N1) in cell culture. Antibiot Khimioter. 2007;52(11-12):18-20. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

