Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D03ZZK
|
|||
| Former ID |
DAP000421
|
|||
| Drug Name |
Fluocinonide
|
|||
| Synonyms |
Bestasone; Biscosal; Cortalar; Fluocinolide; Fluocinonido; Fluocinonidum; Fluonex; Fluzon; Lidex; Lonide; Lyderm;Metosyn; Straderm; Topsymin; Topsyn; Vanos; Fluocinolide acetate; Fluocinolone acetonide acetate; Fluocinonide Emulsified Base; Fluocinonide FAPG; Lidex E; Synalar acetate; Fluocinolone acetonide 21-acetate; Fluocinonido [INN-Spanish]; Fluocinonidum [INN-Latin]; Fluonex (TN); LIDEX (TN); LIDEX-E; Lidex (TN); Lonide (TN); Lyderm (TN); Vanos (TN); Lidex-E (TN); Fluocinonide (JP15/USP/INN); Fluocinonide [USAN:BAN:INN:JAN]; Fluocinonide [USAN:INN:BAN:JAN]; Pregna-1,4-diene-3,20-dione, 6alpha,9-difluoro-11beta,16alpha,17,21-tetrahydroxy-, cyclic 16,17-acetal with acetone, 21-acetate; Pregna-1,4-diene-3,20-dione, 6alpha,9-difluoro-11beta,16alpha,17,21-tetrahydroxy-, cyclic 16,17-acetal with acetone, 21-acetate (8CI); Pregna-1,4-diene-3,20-dione, 6-alpha, 9-difluoro-11-beta,16-alpha,17,21-tetrahydroxy-, cyclic17-acetal with acetone, 21-acetate; 6alpha,9-Difluoro-11beta,16alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione, cyclic 16,17-acetal with acetone, 21-acetate
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Inflammation [ICD-11: 1A00-CA43.1] | Approved | [1], [2] | |
| Therapeutic Class |
Antiinflammatory Agents
|
|||
| Company |
Taro Pharmaceuticals Inc
|
|||
| Structure |
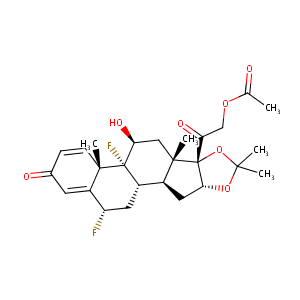 |
Download2D MOL |
||
| Formula |
C26H32F2O7
|
|||
| Canonical SMILES |
CC(=O)OCC(=O)C12C(CC3C1(CC(C4(C3CC(C5=CC(=O)C=CC54C)F)F)O)C)OC(O2)(C)C
|
|||
| InChI |
1S/C26H32F2O7/c1-13(29)33-12-20(32)26-21(34-22(2,3)35-26)10-15-16-9-18(27)17-8-14(30)6-7-23(17,4)25(16,28)19(31)11-24(15,26)5/h6-8,15-16,18-19,21,31H,9-12H2,1-5H3/t15-,16-,18-,19-,21+,23-,24-,25-,26+/m0/s1
|
|||
| InChIKey |
WJOHZNCJWYWUJD-IUGZLZTKSA-N
|
|||
| CAS Number |
CAS 356-12-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9220, 855724, 7847391, 7979242, 8156926, 10321975, 11466802, 11467922, 11486523, 12173604, 14835368, 14933065, 25654074, 29228218, 46504523, 47276849, 47500781, 47945071, 48395149, 49698636, 50019429, 50104032, 57325738, 78820947, 85787530, 92125814, 92717057, 99368359, 103556997, 103914444, 104170119, 104321094, 121362590, 121363485, 124758677, 124800848, 126628971, 126654105, 127307277, 134224335, 134337717, 134973110, 135692551, 135984719, 137005250, 139999927, 144115989, 144203989, 152035935, 152344176
|
|||
| ChEBI ID |
CHEBI:5109
|
|||
| ADReCS Drug ID | BADD_D00925 | |||
| SuperDrug ATC ID |
C05AA11; D07AC08
|
|||
| SuperDrug CAS ID |
cas=000356127
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Steroid hormone receptor ERR (ESRR) | Target Info | Modulator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7078). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 019117. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

