Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D04QNO
|
|||
| Former ID |
DNCL003357
|
|||
| Drug Name |
Symbicort
|
|||
| Synonyms |
(22R)-Budesonide; UNII-2HI1006KPH; 51333-22-3; 51372-29-3; 2HI1006KPH; DSSTox_CID_202; DSSTox_RID_75430; DSSTox_GSID_20202; (1~{s},2~{s},4~{r},6~{r},8~{s},9~{s},11~{s},12~{s},13~{r})-9,13-Dimethyl-11-Oxidanyl-8-(2-Oxidanylethanoyl)-6-Propyl-5,7-Dioxapentacyclo[10.8.0.0^{2,9}.0^{4,8}.0^{13,18}]icosa-14,17-Dien-16-One; R-Budesonide; Budesonide-22R; NCGC00016862-01; EINECS 257-161-7; CAS-51333-22-3; BUDESONIDE (11beta,16alpha(R)); SCHEMBL4095; AC1L22VC; CHEMBL2110662
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic obstructive pulmonary disease [ICD-11: CA22; ICD-10: J44, J44.9] | Approved | [1] | |
| Company |
AstraZeneca
|
|||
| Structure |
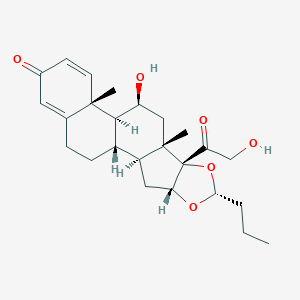 |
Download2D MOL |
||
| Formula |
C25H34O6
|
|||
| Canonical SMILES |
CCCC1OC2CC3C4CCC5=CC(=O)C=CC5(C4C(CC3(C2(O1)C(=O)CO)C)O)C
|
|||
| InChI |
1S/C25H34O6/c1-4-5-21-30-20-11-17-16-7-6-14-10-15(27)8-9-23(14,2)22(16)18(28)12-24(17,3)25(20,31-21)19(29)13-26/h8-10,16-18,20-22,26,28H,4-7,11-13H2,1-3H3/t16-,17-,18-,20+,21+,22+,23-,24-,25+/m0/s1
|
|||
| InChIKey |
VOVIALXJUBGFJZ-VXKMTNQYSA-N
|
|||
| CAS Number |
CAS 51372-29-3
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approves Symbicort for Chronic Obstructive Pulmonary Disease | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

