Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D05UYW
|
|||
| Former ID |
DAP000775
|
|||
| Drug Name |
Dorzolamide
|
|||
| Synonyms |
Trusopt; Dorzolamide (DZA); Dorzolamide (INN); Trusopt (TN); (4S,6S)-4-(ethylamino)-6-methyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide 7,7-dioxide; (4S,6S)-4-(ethylamino)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide; (4S,6S)-4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3-b]thiopyran-2-sulfonic acid amide; (4S,trans)-4-(ethylamino)-6-methyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide 7,7-dioxide; (4S-TRANS)-4-(ETHYLAMINO)-5,6-DIHYDRO-6-METHYL-4H-THIENO(2,3-B)THIOPYRAN-2-SULFONAMIDE-7,7-DIOXIDE
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Open-angle glaucoma [ICD-11: 9C61; ICD-9: 365] | Approved | [1], [2] | |
| Therapeutic Class |
Antihypertensive Agents
|
|||
| Company |
Merck & Co
|
|||
| Structure |
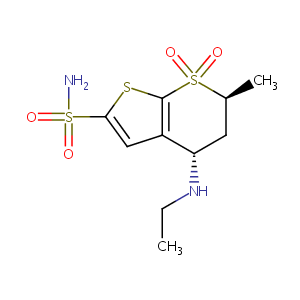 |
Download2D MOL |
||
| Formula |
C10H16N2O4S3
|
|||
| Canonical SMILES |
CCNC1CC(S(=O)(=O)C2=C1C=C(S2)S(=O)(=O)N)C
|
|||
| InChI |
1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1
|
|||
| InChIKey |
IAVUPMFITXYVAF-XPUUQOCRSA-N
|
|||
| CAS Number |
CAS 120279-96-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9183, 7887377, 7979124, 11039340, 11467144, 11468264, 11486852, 14752890, 14752891, 39317865, 46391654, 46506754, 46506976, 46511454, 47216874, 47589091, 47959869, 48334604, 48415919, 49699364, 50445654, 57359110, 85752887, 85787534, 92308962, 93166464, 93308959, 94569219, 96024567, 99313635, 104234231, 113863152, 117889294, 126652186, 131296245, 131858882, 134338060, 136062818, 137005797, 139083143, 162103346, 162180745, 162182114, 174006873, 174526418, 175267119, 176484076, 176484975, 178103416, 179149688
|
|||
| ChEBI ID |
CHEBI:4702
|
|||
| ADReCS Drug ID | BADD_D00006 ; BADD_D00712 ; BADD_D00713 | |||
| SuperDrug ATC ID |
S01EC03
|
|||
| SuperDrug CAS ID |
cas=120279961
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Carbonic anhydrase II (CA-II) | Target Info | Inhibitor | [3] |
| KEGG Pathway | Nitrogen metabolism | |||
| Proximal tubule bicarbonate reclamation | ||||
| Collecting duct acid secretion | ||||
| Gastric acid secretion | ||||
| Pancreatic secretion | ||||
| Bile secretion | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| EGFR1 Signaling Pathway | ||||
| Reactome | Erythrocytes take up carbon dioxide and release oxygen | |||
| Erythrocytes take up oxygen and release carbon dioxide | ||||
| Reversible hydration of carbon dioxide | ||||
| WikiPathways | Reversible Hydration of Carbon Dioxide | |||
| Uptake of Carbon Dioxide and Release of Oxygen by Erythrocytes | ||||
| Uptake of Oxygen and Release of Carbon Dioxide by Erythrocytes | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6810). | |||
| REF 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 3 | Carbonic anhydrase inhibitors. Inhibition studies of a coral secretory isoform by sulfonamides. Bioorg Med Chem. 2009 Jul 15;17(14):5054-8. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

