Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06AAP
|
|||
| Former ID |
DIB002802
|
|||
| Drug Name |
Ipodate
|
|||
| Synonyms |
Oragrafin Calcium; Bilivist; Oragrafin Sodium
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Graves disease [ICD-11: 5A02.0; ICD-10: E05.0, H06.2] | Approved | [1] | |
| Company |
Bracco Diagnostics Inc
|
|||
| Structure |
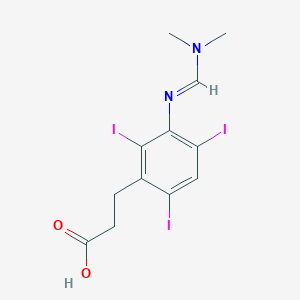 |
Download2D MOL |
||
| Formula |
C12H13I3N2O2
|
|||
| Canonical SMILES |
CN(C)C=NC1=C(C=C(C(=C1I)CCC(=O)O)I)I
|
|||
| InChI |
1S/C12H13I3N2O2/c1-17(2)6-16-12-9(14)5-8(13)7(11(12)15)3-4-10(18)19/h5-6H,3-4H2,1-2H3,(H,18,19)
|
|||
| InChIKey |
YQNFBOJPTAXAKV-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 5587-89-3
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:81496
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

