Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06DLI
|
|||
| Former ID |
DCL000877
|
|||
| Drug Name |
MCI-186
|
|||
| Synonyms |
Edarabone; Edaravone; Methylphenylpyrazolone; Monopyrazolone; Norantipyrine; Norphenazone; Phenylmethylpyrazolone; Radicut; Developer Z; Edaravone [INN]; Phenyl methyl pyrazolone; CDS1_000986; CI Developer 1; IN1263; M0687; MCI 186; Edaravone(jan); Radicut (TN); AE-641/00371017; C.I. Developer 1; Edaravone (JAN/INN); (MCI-186); 1-Fenyl-3-methyl-2-pyrazolin-5-on; 1-Fenyl-3-methyl-2-pyrazolin-5-on [Czech]; 1-Phenyl-3-methyl-5-oxo-2-pyrazoline; 1-Phenyl-3-methyl-5-pyrazolone; 1-Phenyl-3-methylpyrazolone; 1-Phenyl-3-methylpyrazolone-5; 2,4-Dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one; 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE; 3-Methyl-1-phenyl-2-pyrazoline-5-one; 3-Methyl-1-phenyl-5-pyrazolone; 3-Methyl-1-phenylpyrazol-5-one; 5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one; 5-methyl-2-phenyl-4H-pyrazol-3-one
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Amyotrophic lateral sclerosis [ICD-11: 8B60.0; ICD-9: 335.2] | Approved | [1] | |
| Company |
Mitsubishi Tanabe
|
|||
| Structure |
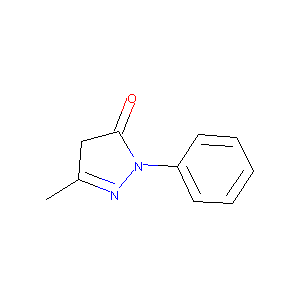 |
Download2D MOL |
||
| Formula |
C10H10N2O
|
|||
| Canonical SMILES |
CC1=NN(C(=O)C1)C2=CC=CC=C2
|
|||
| InChI |
1S/C10H10N2O/c1-8-7-10(13)12(11-8)9-5-3-2-4-6-9/h2-6H,7H2,1H3
|
|||
| InChIKey |
QELUYTUMUWHWMC-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 89-25-8
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
66965, 69252, 87060, 583397, 855774, 3133945, 5663208, 7848615, 8150018, 8152519, 10537545, 11113339, 11120318, 11120806, 11121294, 11121793, 11122273, 11336193, 11337201, 11361432, 11362989, 11363161, 11365551, 11365723, 11368113, 11368285, 11371129, 11371130, 11371920, 11373714, 11374640, 11376275, 11376447, 11384009, 11462404, 11485244, 11489390, 11490786, 11492905, 11494081, 11512560, 12014003, 15219562, 16959864, 17389763, 22389521, 24885706, 24897140, 25622932, 26612506
|
|||
| ChEBI ID |
CHEBI:31530
|
|||
| ADReCS Drug ID | BADD_D02508 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||
| REF 2 | Emerging disease-modifying therapies for the treatment of motor neuron disease/amyotropic lateral sclerosis. Expert Opin Emerg Drugs. 2007 May;12(2):229-52. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

