Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D06PTA
|
|||
| Former ID |
DNCL002808
|
|||
| Drug Name |
Levosalbutamol/ipratropium
|
|||
| Synonyms |
8-Azoniabicyclo(3.2.1)octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, (endo,syn)-; 3-(3-Hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-8-azoniabicyclo(3.2.1)octane (3-endo,8-syn)-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic obstructive pulmonary disease [ICD-11: CA22; ICD-10: J44, J44.9] | Phase 4 | [1] | |
| Company |
Sunovion Pharmaceuticals
|
|||
| Structure |
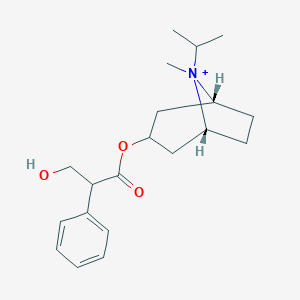 |
Download2D MOL |
||
| Formula |
C20H30NO3+
|
|||
| Canonical SMILES |
CC(C)[N+]1(C2CCC1CC(C2)OC(=O)C(CO)C3=CC=CC=C3)C
|
|||
| InChI |
1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1/t16-,17-,18?,19?,21?/m1/s1
|
|||
| InChIKey |
OEXHQOGQTVQTAT-IPHBXJNRSA-N
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:91661
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT00911651) Study to Assess the Effect of Salbutamol and Ipratropium Bromide in Chronic Obstructive Pulmonary Disease (COPD) Patients. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

