Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07NUL
|
|||
| Former ID |
DIB001623
|
|||
| Drug Name |
PF-06650833
|
|||
| Indication | Lupus [ICD-11: 4A40; ICD-9: 710] | Phase 1 | [1] | |
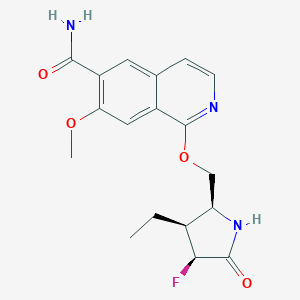
| Structure |
 |
Download2D MOL |
||
| Formula |
C18H20FN3O4
|
|||
| Canonical SMILES |
CCC1C(NC(=O)C1F)COC2=NC=CC3=CC(=C(C=C32)OC)C(=O)N
|
|||
| InChI |
1S/C18H20FN3O4/c1-3-10-13(22-17(24)15(10)19)8-26-18-11-7-14(25-2)12(16(20)23)6-9(11)4-5-21-18/h4-7,10,13,15H,3,8H2,1-2H3,(H2,20,23)(H,22,24)/t10-,13+,15-/m0/s1
|
|||
| InChIKey |
JKDGKIBAOAFRPJ-ZBINZKHDSA-N
|
|||
| CAS Number |
CAS 1817626-54-2
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02224651) Study to Evaluate Safety and Tolerability of Single Ascending Doses of Multiple Formulations of PF-06650833 in Healthy Subjects Under Fasted and Fed Conditions. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

