Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07RGW
|
|||
| Former ID |
DAP000508
|
|||
| Drug Name |
Mephenytoin
|
|||
| Synonyms |
Epiazin; Epilan; Fenantoin; Insulton; Mefenetoin; Mefenitoina; Mephenetoinum; Mephentoin; Mephenytoine; Mephenytoinum; Mesantoin; Mesdontoin; Mesontoin; Methoin; Methylphenetoin; Metydan; Phenantoin; Phenylethylmethylhydantoin; Sacerno; Sedantional; Sedantoin; Sedantoinal; Triantoin; Ydantoin; Methyl Phenetoin; Methyl hydantoin; MU2276000; Gerot-epilan; L-Mephenytoin; Mefenitoina [INN-Spanish]; Mephenytoin [USAN:INN]; Mephenytoine [INN-French]; Mephenytoinum [INN-Latin]; Mesantoin (TN); Phenetoin, Methyl;Mephenytoin (USP/INN); L-3-Methyl-5-ethyl-5-phenylhydantoin; (+-)-5-Ethyl-3-methyl-5-phenylhydantoin; (+-)-Mephenytoin; (-)-5-Ethyl-3-methyl-5-phenylhydantoin; 3-Ethylnirvanol; 3-Methyl-5, 5-ethylphenylhydantoin; 3-Methyl-5,5-ethylphenylhydantoin; 3-Methyl-5,5-phenylethylhydantoin; 3-Methyl-5-ethyl-5-phenylh; 3-Methyl-5-ethyl-5-phenylhydantoin; 5 Ethyl 3 Methyl 5 Phenylhydantoin; 5-Ethyl-3-methyl-5-phenyl-2,4(3H,5H)-imidazoledione; 5-Ethyl-3-methyl-5-phenyl-2,4-imidazolidinedione; 5-Ethyl-3-methyl-5-phenylhydantoin; 5-Ethyl-3-methyl-5-phenylimidazolidin-2,4-dione; 5-Ethyl-5-fenyl-3-methylhydantoin; 5-Ethyl-5-fenyl-3-methylhydantoin [Czech]; 5-ethyl-3-methyl-5-phenylimidazolidine-2,4-dione
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Epilepsy [ICD-11: 8A60-8A68] | Approved | [1], [2] | |
| Therapeutic Class |
Anticonvulsants
|
|||
| Company |
Norvatis Phamaceuticals Corporation
|
|||
| Structure |
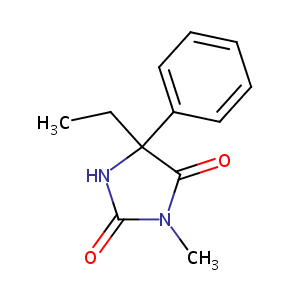 |
Download2D MOL |
||
| Formula |
C12H14N2O2
|
|||
| Canonical SMILES |
CCC1(C(=O)N(C(=O)N1)C)C2=CC=CC=C2
|
|||
| InChI |
1S/C12H14N2O2/c1-3-12(9-7-5-4-6-8-9)10(15)14(2)11(16)13-12/h4-8H,3H2,1-2H3,(H,13,16)
|
|||
| InChIKey |
GMHKMTDVRCWUDX-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 50-12-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
92176, 220439, 4359670, 7847441, 7872066, 8150040, 8152547, 10524271, 11336112, 11361351, 11364388, 11366950, 11369512, 11374646, 11377674, 11462323, 11467136, 11468256, 11486824, 11492908, 11495308, 14773490, 29217682, 29223171, 46508677, 47291207, 47662362, 47959838, 47959839, 48035210, 48185062, 48416216, 49698809, 49703443, 49977224, 49977225, 50111722, 53789258, 56463168, 57322121, 79894519, 85788588, 92126062, 92308878, 92714624, 99302025, 103224438, 103914622, 104305314, 121363733
|
|||
| ChEBI ID |
CHEBI:6757
|
|||
| ADReCS Drug ID | BADD_D01386 | |||
| SuperDrug ATC ID |
N03AB04
|
|||
| SuperDrug CAS ID |
cas=000050124
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Voltage-gated sodium channel alpha Nav1.5 (SCN5A) | Target Info | Blocker | [3] |
| KEGG Pathway | Adrenergic signaling in cardiomyocytes | |||
| Pathwhiz Pathway | Muscle/Heart Contraction | |||
| Reactome | Interaction between L1 and Ankyrins | |||
| WikiPathways | SIDS Susceptibility Pathways | |||
| Cardiac Progenitor Differentiation | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7223). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 006008. | |||
| REF 3 | Lacosamide: a new approach to target voltage-gated sodium currents in epileptic disorders. CNS Drugs. 2009;23(7):555-68. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

