Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D07VDZ
|
|||
| Former ID |
DAP000137
|
|||
| Drug Name |
Topiramate
|
|||
| Synonyms |
Epitoma; Epitomax; TOR; Tipiramate; Tipiramato; Topamac; Topamax; Topimax; Topina; Topiramato; Topiramatum; Topomax; Cilag brandof topiramate; Janssen brand of topiramate; Ortho brand of topiramate; Tipiramate [French]; Tipiramato [Spanish]; Topamax Sprinkle; Topiramate tablet; Topiramatum [Latin]; Topiramic acid; McN 4853; RWJ 17021; KS-1122; KW-6485; McN-4853; RWJ-17021; Topamax (TN); Topamax, Topiramate; Topiramate (TPM); Topiramate / Placebo; Topiramato [INN-Spanish]; Topiramatum [INN-Latin]; USL-255; RWJ-17021-000; Topiramate [USAN:BAN:INN]; Topiramate (JAN/USAN/INN); Beta-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, sulfamate; Beta-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, sulfamate (9CI);Beta.-D-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, 1-sulfamate; [(3aS,5aR,8aR,8bS)-2,2,7,7-tetramethyltetrahydro-3aH-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-3a-yl]methyl sulfamate; 2,3-4,5-bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate; 2,3:4,5-Bis-O-(1-methylethylidene) .beta.-D-fructopyranose sulfamate; 2,3:4,5-Bis-O-(1-methylethylidene)-36-D-fructo-pyranose sulfamate; 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate; 2,3:4,5-Di-O-isopropylidene-(beta)-D-fructopyranose sulfamate; 2,3:4,5-Di-O-isopropylidene-beta-D-fructopyranose sulfamate; 5H-Bis[1,3]dioxolo[4,5-b:4',5'-d]pyran, beta
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Alcohol dependence [ICD-11: 6C40.2; ICD-10: F10.2] | Approved | [1], [2], [3] | |
| Epilepsy [ICD-11: 8A60-8A68] | Approved | [1], [2], [3] | ||
| Seizure disorder [ICD-11: 8A6Z; ICD-9: 345.9, 780.3] | Investigative | [4] | ||
| Therapeutic Class |
Anticonvulsants
|
|||
| Company |
Ortho-McNell Pharmaceutical
|
|||
| Structure |
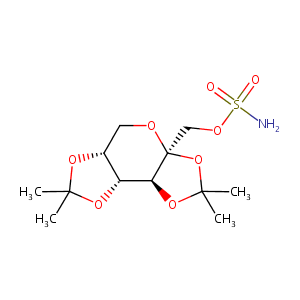 |
Download2D MOL |
||
| Formula |
C12H21NO8S
|
|||
| Canonical SMILES |
CC1(OC2COC3(C(C2O1)OC(O3)(C)C)COS(=O)(=O)N)C
|
|||
| InChI |
1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1
|
|||
| InChIKey |
KJADKKWYZYXHBB-XBWDGYHZSA-N
|
|||
| CAS Number |
CAS 97240-79-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9705, 7847603, 7980814, 11039383, 11484731, 11488944, 11528651, 12013555, 14777895, 14924784, 24724627, 25819957, 26612934, 26719832, 39317930, 46386619, 46508334, 46511457, 47277061, 47425755, 48416647, 48764342, 49681704, 49835829, 50070928, 50123385, 53787612, 57359151, 77351910, 85248100, 92124710, 92307910, 92308459, 93167012, 93167145, 97857519, 99437151, 104109398, 104234233, 104971753, 124658936, 124757247, 124800161, 125164051, 125312450, 126525327, 126606833, 126670104, 128389335, 134337459
|
|||
| ChEBI ID |
CHEBI:63631
|
|||
| ADReCS Drug ID | BADD_D02247 | |||
| SuperDrug ATC ID |
N03AX11
|
|||
| SuperDrug CAS ID |
cas=097240794
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Glutamate receptor ionotropic kainate 1 (GRIK1) | Target Info | Antagonist | [5] |
| KEGG Pathway | Neuroactive ligand-receptor interaction | |||
| Glutamatergic synapse | ||||
| Panther Pathway | Huntington disease | |||
| Ionotropic glutamate receptor pathway | ||||
| Metabotropic glutamate receptor group III pathway | ||||
| Metabotropic glutamate receptor group I pathway | ||||
| WikiPathways | Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6849). | |||
| REF 2 | Use of second-generation antiepileptic drugs in the pediatric population. Paediatr Drugs. 2008;10(4):217-54. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 4 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||
| REF 5 | Development of medications for alcohol use disorders: recent advances and ongoing challenges. Expert Opin Emerg Drugs. 2005 May;10(2):323-43. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

