Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D08TRG
|
|||
| Former ID |
DIB014163
|
|||
| Drug Name |
AZD-3241
|
|||
| Synonyms |
Parkinson's disease therapeutic, AstraZeneca
Click to Show/Hide
|
|||
| Indication | Parkinson disease [ICD-11: 8A00.0; ICD-9: 332] | Phase 2 | [1] | |
| Company |
AstraZeneca plc
|
|||
| Structure |
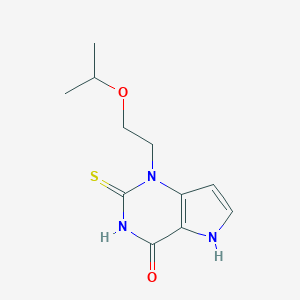 |
Download2D MOL |
||
| Formula |
C11H15N3O2S
|
|||
| Canonical SMILES |
CC(C)OCCN1C2=C(C(=O)NC1=S)NC=C2
|
|||
| InChI |
1S/C11H15N3O2S/c1-7(2)16-6-5-14-8-3-4-12-9(8)10(15)13-11(14)17/h3-4,7,12H,5-6H2,1-2H3,(H,13,15,17)
|
|||
| InChIKey |
FVJCUZCRPIMVLB-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 890655-80-8
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Myeloperoxidase (MPO) | Target Info | Inhibitor | [2] |
| KEGG Pathway | Phagosome | |||
| Transcriptional misregulation in cancer | ||||
| Pathway Interaction Database | C-MYB transcription factor network | |||
| IL23-mediated signaling events | ||||
| WikiPathways | Folate Metabolism | |||
| Vitamin B12 Metabolism | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02388295) AZD3241 PET MSA Trial, Phase 2, Randomized,12 Week Safety and Tolerability Trial With PET in MSA Patients. U.S. National Institutes of Health. | |||
| REF 2 | Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson's disease. Brain. 2015 Sep;138(Pt 9):2687-700. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

