Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09PZO
|
|||
| Former ID |
DAP000997
|
|||
| Drug Name |
Idoxuridine
|
|||
| Synonyms |
Emanil; Herpesil; Herpidu; Idossuridina; Iododeoxyuridine; Kerecide; Ophthalmadine; Synmiol; ID2; LT00440811; SKF 14287; Dendrid (TN); HERPLEX (TN); Herpe-Gel; Herpes-Gel; Iododeoxyuridine-125I; Oftan-IDU; SK&F 14287; SK&F-14287; Idoxuridine (JP15/USP/INN); Idoxuridine [USAN:INN:BAN:JAN]; 1-(2-Deoxy-beta-D-ribofuranosyl)-5-iodouracil; 1-(2-deoxypentofuranosyl)-4-hydroxy-5-iodopyrimidin-2(1H)-one; 1-(2-deoxypentofuranosyl)-5-iodopyrimidine-2,4(1h,3h)-dione; 1-(4-Hydroxy-5-hydroxymethyltetrahydrofuran-2-yl)-5-iodo-1H-pyrimidine-2,4-dione; 1-[(2R,4R,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione; 1-[(2R,4R,5S)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione; 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione; 1-[(2R,4S,5S)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione; 1-[(2R,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione; 1-[(2S,4R,5S)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione; 1-[(2S,4S,5S)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione; 1-[4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-iodopyrimidine-2,4-dione; 1-beta-D-2'-Deoxyribofuranosyl-5-iodouracil; 1.beta.-D-2'-Deoxyribofuranosyl-5-iodouracil; 1beta-D-2'-Deoxyribofuranosyl-5-iodouracil; 2'-Deoxy-5-(iodo-125I)uridine; 2'-deoxy-5-(125i)iodouridine; 5-(125-I)-Iodo-2-deoxyuridine; 5-I-2'-dUrd; 5-Iododesoxyuridine; 5IdU
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Herpes simplex virus infection [ICD-11: 1F00; ICD-10: B00; ICD-9: 54] | Approved | [1] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
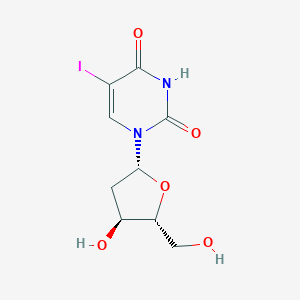 |
Download2D MOL |
||
| Formula |
C9H11IN2O5
|
|||
| Canonical SMILES |
C1C(C(OC1N2C=C(C(=O)NC2=O)I)CO)O
|
|||
| InChI |
1S/C9H11IN2O5/c10-4-2-12(9(16)11-8(4)15)7-1-5(14)6(3-13)17-7/h2,5-7,13-14H,1,3H2,(H,11,15,16)/t5-,6+,7+/m0/s1
|
|||
| InChIKey |
XQFRJNBWHJMXHO-RRKCRQDMSA-N
|
|||
| CAS Number |
CAS 54-42-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:147675
|
|||
| ADReCS Drug ID | BADD_D01131 | |||
| SuperDrug ATC ID |
D06BB01; J05AB02; S01AD01
|
|||
| SuperDrug CAS ID |
cas=000054422
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides fragilis nontoxigenic
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Bacteroides fragilis nontoxigenic was decreased by Idoxuridine (adjusted p-values: 7.14E-03). | |||
|
Studied Microbe: Bacteroides ovatus
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Bacteroides ovatus was decreased by Idoxuridine (adjusted p-values: 3.53E-04). | |||
|
Studied Microbe: Odoribacter splanchnicus
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Odoribacter splanchnicus was decreased by Idoxuridine (adjusted p-values: 1.12E-03). | |||
|
Studied Microbe: Prevotella copri
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Prevotella copri was decreased by Idoxuridine (adjusted p-values: 6.33E-03). | |||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Enterobacterales | ||||
|
Studied Microbe: Escherichia coli ED1a
Show/Hide Hierarchy
|
[2] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Escherichia coli ED1a was decreased by Idoxuridine (adjusted p-values: 8.43E-04). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Virus Deoxyribonucleic acid (Viru DNA) | Target Info | Binder | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 014169. | |||
| REF 2 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 3 | Radiopharmaceuticals (Strontium 89) and radiosensitizers (idoxuridine). J Intraven Nurs. 1998 Nov-Dec;21(6):335-7. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

