Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09UZL
|
|||
| Former ID |
DNCL003786
|
|||
| Drug Name |
TAK-063
|
|||
| Indication | Schizophrenia [ICD-11: 6A20] | Phase 2 | [1] | |
| Company |
Takeda
|
|||
| Structure |
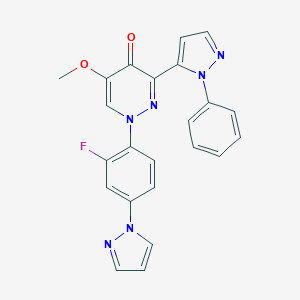 |
Download2D MOL |
||
| Formula |
C23H17FN6O2
|
|||
| Canonical SMILES |
COC1=CN(N=C(C1=O)C2=CC=NN2C3=CC=CC=C3)C4=C(C=C(C=C4)N5C=CC=N5)F
|
|||
| InChI |
1S/C23H17FN6O2/c1-32-21-15-29(19-9-8-17(14-18(19)24)28-13-5-11-25-28)27-22(23(21)31)20-10-12-26-30(20)16-6-3-2-4-7-16/h2-15H,1H3
|
|||
| InChIKey |
KVHRYLNQDWXAGI-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1238697-26-1
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Phosphodiesterase 10A (PDE10) | Target Info | Inhibitor | [2] |
| KEGG Pathway | Purine metabolism | |||
| Morphine addiction | ||||
| Pathwhiz Pathway | Purine Metabolism | |||
| Reactome | cGMP effects | |||
| G alpha (s) signalling events | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02477020) A Phase 2 Efficacy and Safety Study of TAK-063 in Participants With an Acute Exacerbation of Schizophrenia. | |||
| REF 2 | Characterization of Binding and Inhibitory Properties of TAK-063, a Novel Phosphodiesterase 10A Inhibitor. PLoS One. 2015; 10(3): e0122197. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

