Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D09WKB
|
|||
| Former ID |
DAP001274
|
|||
| Drug Name |
Anisindione
|
|||
| Synonyms |
Anisindiona; Anisindionum; Miradon; Unidone; Anisin indandione; SPE 2792; Anisindiona [INN-Spanish]; Anisindione (INN); Anisindione [INN:BAN]; Anisindionum [INN-Latin]; Miradon (TN); 2-(4-Methoxy-phenyl)-indan-1,3-dione; 2-(4-Methoxyphenyl)-1H-indene-1,3(2H)-dione; 2-(4-Methoxyphenyl)indan-1,3-dione; 2-(4-methoxyphenyl)indene-1,3-dione; 2-(p-Methoxyphenyl)-1,3-indandione; 2-(p-Methoxyphenyl)indane-1,3-dione; 2-[4-(methyloxy)phenyl]-1H-indene-1,3(2H)-dione; 2-p-Anisyl-1,3-indandione; 2-para-Anisyl-1,3-indandione
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Coagulation defect [ICD-11: 3B10.0; ICD-10: I80-I82] | Approved | [1], [2] | |
| Therapeutic Class |
Anticoagulants
|
|||
| Structure |
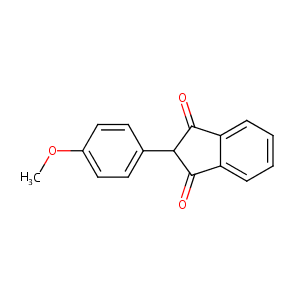 |
Download2D MOL |
||
| Formula |
C16H12O3
|
|||
| Canonical SMILES |
COC1=CC=C(C=C1)C2C(=O)C3=CC=CC=C3C2=O
|
|||
| InChI |
1S/C16H12O3/c1-19-11-8-6-10(7-9-11)14-15(17)12-4-2-3-5-13(12)16(14)18/h2-9,14H,1H3
|
|||
| InChIKey |
XRCFXMGQEVUZFC-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 117-37-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
3150799, 5141622, 7907525, 8149855, 8151491, 10505123, 11335511, 11360750, 11364249, 11366811, 11369373, 11372509, 11374125, 11377535, 11461722, 11484150, 11488243, 11491340, 11492538, 11495169, 14729672, 15391997, 26537315, 26612327, 26679795, 26748631, 26748632, 29221375, 46504660, 47291049, 47515233, 48035021, 48184919, 48415571, 49982247, 50086377, 50107437, 50609859, 51091788, 57288748, 57321195, 58000062, 85333593, 92124482, 92307614, 99301424, 103194851, 104121859, 104299892, 105534934
|
|||
| ChEBI ID |
CHEBI:133809
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6960). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 010909. | |||
| REF 3 | Anticoagulation with anisindione in a patient with a warfarin-induced skin eruption. Pharmacotherapy. 2003 Apr;23(4):533-6. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

