Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0G5BK
|
|||
| Former ID |
DIB004842
|
|||
| Drug Name |
Octafluoropropane perflutren lipid microsphere
|
|||
| Synonyms |
Definity; Luminity; Octafluoropropane; Perflutren; Perflutren lipid microsphere; DM-115; DMP-115; FS-069; FS-69; MRX-115; YM-454; Perflutren (Aerosomes), Lantheus; Perflutren (Aerosomes), Bristol-Myers Squibb Medical Imaging; Perflutren (Aerosomes), ImaRx/DuPont
Click to Show/Hide
|
|||
| Indication | Heart disease [ICD-11: BA41-BA42; ICD-9: 390-429] | Approved | [1], [2] | |
| Company |
Molecular Biosystems Inc; ImaRx Therapeutics Inc
|
|||
| Structure |
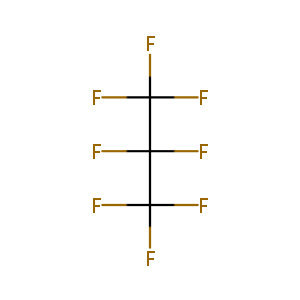 |
Download2D MOL |
||
| ADReCS Drug ID | BADD_D01734 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | ClinicalTrials.gov (NCT00401687) DMP115 in Patients With an Ejection Fraction Between 25%-40% to Evaluate the Use of Contrast Echocardiography to Assess Heart Function. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

