Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0JL2K
|
|||
| Former ID |
DAP001005
|
|||
| Drug Name |
Grepafloxacin
|
|||
| Synonyms |
Raxar; Grepafloxacin [INN]; Grepafloxacin (unspecified); Raxar (TN); (+-)-1-Cyclopropyl-6-fluoro-1,4-dihydro-5-methyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-Cyclopropyl-6-fluoro-1,4-dihydro-5-methyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-cyclopropyl-6-fluoro-5-methyl-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic bronchitis [ICD-11: CA20.1; ICD-10: J41, J42] | Approved | [1] | |
| Bacterial infection [ICD-11: 1A00-1C4Z; ICD-10: A00-B99] | Withdrawn from market | [2] | ||
| Therapeutic Class |
Antibiotics
|
|||
| Company |
GlaxoSmithKline
|
|||
| Structure |
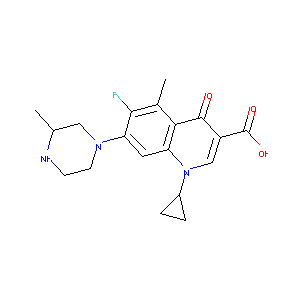 |
Download2D MOL |
||
| Formula |
C19H22FN3O3
|
|||
| Canonical SMILES |
CC1CN(CCN1)C2=C(C(=C3C(=C2)N(C=C(C3=O)C(=O)O)C4CC4)C)F
|
|||
| InChI |
1S/C19H22FN3O3/c1-10-8-22(6-5-21-10)15-7-14-16(11(2)17(15)20)18(24)13(19(25)26)9-23(14)12-3-4-12/h7,9-10,12,21H,3-6,8H2,1-2H3,(H,25,26)
|
|||
| InChIKey |
AIJTTZAVMXIJGM-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 119914-60-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
13542, 603312, 6610845, 8195206, 14852426, 43128629, 46507253, 50042505, 50112780, 50471882, 53788063, 57318735, 103179577, 104011329, 104353024, 124766026, 125536480, 127411146, 134338274, 135029665, 137044453, 143019201, 160963712, 162793470, 163693542, 163852853, 179148061, 184545887, 184546334, 223439875, 224916848, 226420671, 241036011, 242059760
|
|||
| ChEBI ID |
CHEBI:5543
|
|||
| SuperDrug ATC ID |
J01MA11
|
|||
| SuperDrug CAS ID |
cas=119914602
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Bacterial DNA gyrase (Bact gyrase) | Target Info | Modulator | [3] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 020695. | |||
| REF 3 | Grepafloxacin, a dimethyl derivative of ciprofloxacin, acts preferentially through gyrase in Streptococcus pneumoniae: role of the C-5 group in tar... Antimicrob Agents Chemother. 2002 Feb;46(2):582-5. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

