Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0K8CI
|
|||
| Former ID |
DNC001069
|
|||
| Drug Name |
Otilonium bromide
|
|||
| Synonyms |
Otilonium Bromide; 26095-59-0; Octylonium bromide; Spasmomen; Octylonium; Otilonium (bromide); SP63; Ottilonio bromuro [Italian]; UNII-21HN3N72PV; Otilonium bromide [INN:BAN]; Otilonii bromidum [INN-Latin]; Bromure d'otilonium [INN-French]; Bromuro de otilonio [INN-Spanish]; EINECS 247-457-4; 21HN3N72PV; Diethyl(2-hydroxyethyl)methylammonium bromide p-(o-(octyloxy)benzamido)benzoate; Ethanaminium, N,N-diethyl-N-methyl-2-((4-((2-(octyloxy)benzoyl)amino)benzoyl)oxy)-, bromide; DSSTox_CID_26357; DSSTox_RID_81559
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Gastric motility disorder [ICD-11: DA21; ICD-10: K22.4] | Approved | [1] | |
| Company |
Menarini
|
|||
| Structure |
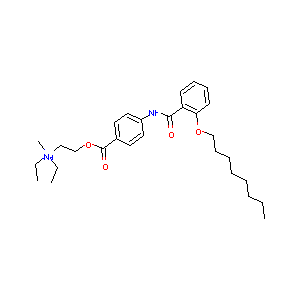 |
Download2D MOL |
||
| Formula |
C29H43BrN2O4
|
|||
| Canonical SMILES |
CCCCCCCCOC1=CC=CC=C1C(=O)NC2=CC=C(C=C2)C(=O)OCC[N+](C)(CC)CC.[Br-]
|
|||
| InChI |
1S/C29H42N2O4.BrH/c1-5-8-9-10-11-14-22-34-27-16-13-12-15-26(27)28(32)30-25-19-17-24(18-20-25)29(33)35-23-21-31(4,6-2)7-3;/h12-13,15-20H,5-11,14,21-23H2,1-4H3;1H
|
|||
| InChIKey |
VWZPIJGXYWHBOW-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 26095-59-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
8195011, 14861886, 43128316, 49867396, 51091425, 57318519, 80288609, 85088345, 85174599, 91615433, 97302044, 103836341, 104352072, 117370323, 118843737, 125346362, 126668384, 134222557, 135029156, 135544004, 144205612, 151972496, 160687004, 162009755, 162092335, 162185199, 163122775, 164814318, 170466098, 174528385, 184545514, 187072408, 198981890, 210279657, 210281980, 223435291, 223666289, 223705228, 226483283, 252215976, 252359360, 252418757
|
|||
| SuperDrug ATC ID |
A03AB06
|
|||
| SuperDrug CAS ID |
cas=026095590
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Voltage-gated L-type calcium channel (L-CaC) | Target Info | Blocker | [2] |
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Emerging drugs for irritable bowel syndrome. Expert Opin Emerg Drugs. 2006 May;11(2):293-313. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

