Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0M0AM
|
|||
| Former ID |
DAP001116
|
|||
| Drug Name |
Glycopyrrolate
|
|||
| Synonyms |
GLYCOPYRROLATE; 596-51-0; Glycopyrrolate bromide; Robinul; Gastrodyn; Tarodyl; Nodapton; Tarodyn; Asecryl; Copyrrolate; Cuvposa; Glycopyrronii bromidum; AHR-504; ROBINUL FORTE; Robinal; Robanul; Bromuro de glicopirronio; Bromure de glycopyrronium; NVA-237; AHR 504; Glycopyrronii bromidum [INN-Latin]; EINECS 209-887-0; Bromure de glycopyrronium [INN-French]; Bromuro de glicopirronio [INN-Spanish]; 3-Hydroxy-1,1-dimethylpyrrolidinium bromide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Anaesthesia [ICD-11: 9A78.6; ICD-9: 338] | Approved | [1], [2] | |
| Chronic obstructive pulmonary disease [ICD-11: CA22; ICD-10: J44, J44.9] | Approved | [3], [4] | ||
| Therapeutic Class |
Anticholinergic Agents
|
|||
| Company |
Novartis
|
|||
| Structure |
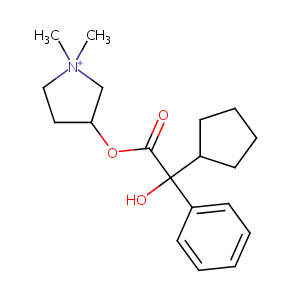 |
Download2D MOL |
||
| Formula |
C19H28BrNO3
|
|||
| Canonical SMILES |
C[N+]1(CCC(C1)OC(=O)C(C2CCCC2)(C3=CC=CC=C3)O)C.[Br-]
|
|||
| InChI |
1S/C19H28NO3.BrH/c1-20(2)13-12-17(14-20)23-18(21)19(22,16-10-6-7-11-16)15-8-4-3-5-9-15;/h3-5,8-9,16-17,22H,6-7,10-14H2,1-2H3;1H/q+1;/p-1
|
|||
| InChIKey |
VPNYRYCIDCJBOM-UHFFFAOYSA-M
|
|||
| CAS Number |
CAS 596-51-0
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
794727, 4582008, 7351415, 8152218, 11466774, 11467894, 11486407, 29222626, 46509133, 47275736, 47720778, 47869842, 48318623, 49699159, 50124171, 57321838, 80767354, 85209742, 103825408, 104234350, 104303677, 117554810, 124893611, 125081928, 127431325, 136023846, 137008742, 141652405, 141652414, 162108778, 162459186, 163366469, 163368548, 179150741, 184685744, 187051762, 224504317, 226501389, 246480751, 247320628
|
|||
| ChEBI ID |
CHEBI:90972
|
|||
| ADReCS Drug ID | BADD_D01034 ; BADD_D02432 | |||
| SuperDrug ATC ID |
A03AB02; R03BB06
|
|||
| SuperDrug CAS ID |
cas=000596510
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7459). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040568. | |||
| REF 3 | Clinical pipeline report, company report or official report of Novartis. | |||
| REF 4 | ClinicalTrials.gov (NCT02181023) Acute Effect of Aclidinium on Hyperinflation and Ventilation Inhomogeneity in Severe COPD Patients. U.S. National Institutes of Health. | |||
| REF 5 | Autonomic cardiovascular control during a novel pharmacologic alternative to ganglionic blockade. Clin Pharmacol Ther. 2008 May;83(5):692-701. | |||
| REF 6 | A pharmacological profile of glycopyrrolate: interactions at the muscarinic acetylcholine receptor. Gen Pharmacol. 1992 Nov;23(6):1165-70. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

