Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0NI0C
|
|||
| Former ID |
DAP000642
|
|||
| Drug Name |
Vidarabine
|
|||
| Synonyms |
Araadenosine; Arabinosyladenine; Armes; RAB; Spongoadenosine; VIRDARABINE; Vidarabin; Vidarabina; Vidarabinum; Xylosyladenine; Adenine arabinoside; Adenine xyloside; Adenosine arabinose; Ara A; Arabinoside adenine; Arabinosyl adenine; Vidarabina [DCIT]; Vidarabine anhydrous; Vira ATM; Xylosyl A; A 9251; ARA-A NSC 247519; Alpha-Ara A; Ara-A; Arabinosyl-adenine; Arasena-A; Armes (TN); Beta-Ara A; Vidarabine (JAN); Vira-A; Adenosine-8-14C; Vira-A, Vidarabine; ADENOSINE, U.S.P.; (+)-Cyclaradine; 9-Arabinosyladenine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Herpes simplex virus infection [ICD-11: 1F00; ICD-10: B00; ICD-9: 54] | Approved | [1], [2] | |
| Therapeutic Class |
Antiviral Agents
|
|||
| Structure |
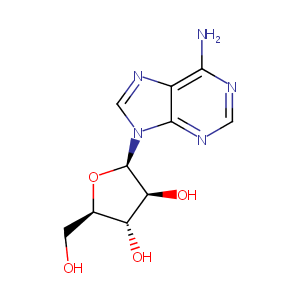 |
Download2D MOL |
||
| Formula |
C10H13N5O4
|
|||
| Canonical SMILES |
C1=NC(=C2C(=N1)N(C=N2)C3C(C(C(O3)CO)O)O)N
|
|||
| InChI |
1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7+,10-/m1/s1
|
|||
| InChIKey |
OIRDTQYFTABQOQ-UHTZMRCNSA-N
|
|||
| CAS Number |
CAS 5536-17-4
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
602757, 1493762, 7890208, 7980879, 8149583, 8165985, 11335366, 11360605, 11364830, 11367392, 11369954, 11371600, 11374363, 11378122, 11461577, 11485227, 11489152, 11490396, 11492597, 11495708, 14799159, 14848243, 24891019, 26611977, 26679886, 26697127, 26740884, 29289080, 46506630, 47193870, 47207955, 47217036, 47365446, 47515571, 47515572, 47959993, 48334764, 49865027, 50123560, 53788311, 56324720, 57264435, 57304786, 57330956, 76747708, 91011419, 92124449, 92307566, 92309102, 99301279
|
|||
| ChEBI ID |
CHEBI:45327
|
|||
| ADReCS Drug ID | BADD_D02354 | |||
| SuperDrug ATC ID |
J05AB03; S01AD06
|
|||
| SuperDrug CAS ID |
cas=005536174
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Enterobacterales | ||||
|
Studied Microbe: Escherichia coli Nissle 1917
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Metabolic Effect | Decrease activity | |||
| Description | Vidarabine can be metabolized by Escherichia coli Nissle 1917, which results in the decrease of the drug's activity. | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adenosine A2b receptor (ADORA2B) | Target Info | Modulator | [4] |
| KEGG Pathway | Rap1 signaling pathway | |||
| Calcium signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Vascular smooth muscle contraction | ||||
| Alcoholism | ||||
| NetPath Pathway | TGF_beta_Receptor Signaling Pathway | |||
| TCR Signaling Pathway | ||||
| Pathwhiz Pathway | Intracellular Signalling Through Adenosine Receptor A2b and Adenosine | |||
| Pathway Interaction Database | C-MYB transcription factor network | |||
| Reactome | Adenosine P1 receptors | |||
| G alpha (s) signalling events | ||||
| Surfactant metabolism | ||||
| WikiPathways | Nucleotide GPCRs | |||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4806). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 050486. | |||
| REF 3 | Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep. 2015 Sep 29;5:14554. | |||
| REF 4 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

