Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0P1FO
|
|||
| Former ID |
DAP000207
|
|||
| Drug Name |
Marinol
|
|||
| Synonyms |
Dronabinol; TETRAHYDROCANNABINOL; delta9-Tetrahydrocannabinol; delta9-THC; Deltanyne; Dronabinolum; delta-9-tetrahydrocannabinol; Abbott 40566; delta-9-THC; delta(9)-THC; 1972/8/3; delta1-THC; delta(sup 1)-Thc; delta(sup 9)-Thc; 1-trans-delta-9-Tetrahydrocannabinol; THC; Namisol; Dronabinolum [Latin]; 9-tetrahydrocannabinol; delta(9)-Tetrahydrocannabinol; delta(1)-Tetrahydrocannabinol; (-)-delta9-trans-Tetrahydrocannabinol; delta1-Tetrahydrocannabinol; 1-trans-delta9-Tetrahydrocannabinol; delta(9)-Tetrahydrocannibinol; SP; Compassia; Ganja; Hashish; Marincap; MaxEPA; Omegaven; Promega; Relivar; Sonergx; Tetranabinex; Cannabis resin; Drona binol; QCD 84924; SP 104; Tetrahydrocannabinol delta9; CAT-310; DELTA1-THC; DELTA1-Tetrahydrocannabinol;DELTA9-THC; DRG-0138; Delta-THC; Delta1-THC; Delta1-Tetrahydrocannabinol; Delta9-THC; Delta9-Tetrahydrocannabinol; Delta9-Tetrahydrocannabinol solution; Dronabinol [USAN:INN]; Marinol (TN); Pro-Mega;QCD-84924; Trans-tetrahydrocannabinol; DELTA9-trans-Tetrahydrocannabinol; Delta(1)-THC; Delta(1)-Tetrahydrocannabinol; Delta(9)-THC; Delta(9)-Tetrahydrocannabinol; Delta(9)-Tetrahydrocannibinol; Delta(sup 1)-Tetrahydrocannabinol; Delta(sup 1)-Thc; Delta(sup 9)-Tetrahydrocannabinol; Delta(sup 9)-Thc; Delta(sup9)-THC; Delta-9-THC; Delta-9-tetrahydrocannabinol; Delta1-Tetrahydrocannabinol (VAN); Delta9-Tetrahydrocannabinol (VAN); Delta9-trans-Tetrahydrocannabinol; Dronabinol (USP/INN); Omega-3-Fatty acid; THC-delta-9; Trans-delta9-Tetrahydrocannabinol; Cannabinol, tetrahydro-(6CI); Fish oils, n-3 fatty acid-high; Fish oils, omega-3 fatty acid-high; L-delta(sup 1)-tetrahydrocannabinol; L-delta1-trans-Tetrahydrocannabinol; L-trans-delta9-Tetrahydrocannabinol; Trans-delta-9-Tetrahydrocannabinol; Cannabinol, Delta1-tetrahydro-(7CI); Fats and Glyceridic oils, fish, n-3 fatty acid-high; L-delta1-Tetrahydrocannabinol; Trans-DELTA9-Tetrahydrocannabinol; L-delta1-trans-Tetrahydrocannabinol;L-trans-delta9-Tetrahydrocannabinol; Tetrahydrocannabinols (-)-delta1-3,4-trans-form; (-)-DELTA1-Tetrahydrocannabinol; (-)-DELTA9-THC; (-)-DELTA9-Tetrahydrocannabinol; (-)-DELTA9-trans-Tetrahydrocannabinol; (-)-delta(sup9)-trans-Tetrahydrocannabinol; (-)-3,4-trans-Delta1-Tetrahydrocannabinol; (-)-delta(sup 1)-3,4-trans-Tetrahydrocannabinol; (-)-delta1-Tetrahydrocannabinol; (-)-delta9-(trans)-Tetrahydrocannabinol; (-)-delta9-Tetrahydrocannabinol; (-)-trans-DELTA9-Tetrahydrocannabinol; (-)-trans-Delta1-Tetrahydrocannabinol; (-)-trans-Delta9-THC; (-)-trans-delta9-Tetrahydrocannabinol; (L)-delta1-Tetrahydrocannabinol; (l)-delta(sup 1)-Tetrahydrocannabinol; (l)-delta1-Tetrahydrocannabinol; 1-trans-delta(sup9)-tetrahydrocannabinol; 1-trans-D9-Tetrahydrocannabinol; 1-trans-delta(sup 9)-Tetrahydrocannabinol; 14C-DELTA1-Tetrahydrocannabinol; 6H-Dibenzo; 9-delta-Tetrahydrocannabinol; 9-ene-Tetrahydrocannabinol; Delta(9)-tetrahydrocannabinol
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Anorexia nervosa cachexia [ICD-11: 6B80; ICD-10: R63.0; ICD-9: 307.1] | Approved | [1] | |
| Chemotherapy-induced nausea [ICD-11: MD90; ICD-10: R11] | Phase 2/3 | [2] | ||
| Therapeutic Class |
Analgesics
|
|||
| Company |
Unimed Pharmaceuticals
|
|||
| Structure |
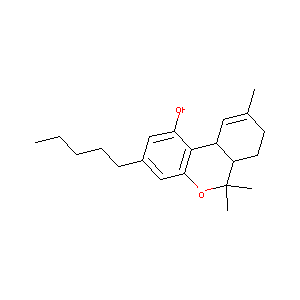 |
Download2D MOL |
||
| Formula |
C21H30O2
|
|||
| Canonical SMILES |
CCCCCC1=CC(=C2C3C=C(CCC3C(OC2=C1)(C)C)C)O
|
|||
| InChI |
1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1
|
|||
| InChIKey |
CYQFCXCEBYINGO-IAGOWNOFSA-N
|
|||
| CAS Number |
CAS 1972-08-3
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9186, 423124, 590069, 841255, 7847372, 7980767, 8162055, 12013925, 14776764, 14899056, 24900035, 24900224, 29283967, 46508472, 47289005, 48414516, 48422188, 49681167, 49855137, 53790103, 56311071, 56365468, 57288574, 57329243, 57654672, 68530527, 78512325, 93166585, 103167825, 103920666, 104338871, 113635228, 117426979, 127309746, 127309747, 127309748, 127309749, 127309750, 127309751, 127309752, 127309753, 127340561, 127340562, 127340563, 127340564, 127344438, 127696830, 134337522, 134339937, 134982169
|
|||
| ChEBI ID |
CHEBI:66964
|
|||
| ADReCS Drug ID | BADD_D00729 | |||
| SuperDrug ATC ID |
A04AD10
|
|||
| SuperDrug CAS ID |
cas=001972083
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cannabinoid receptor 1 (CB1) | Target Info | Agonist | [3], [4] |
| KEGG Pathway | Rap1 signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Retrograde endocannabinoid signaling | ||||
| Panther Pathway | Endogenous cannabinoid signaling | |||
| Pathway Interaction Database | N-cadherin signaling events | |||
| Reactome | Class A/1 (Rhodopsin-like receptors) | |||
| G alpha (i) signalling events | ||||
| WikiPathways | GPCRs, Class A Rhodopsin-like | |||
| Small Ligand GPCRs | ||||
| BDNF signaling pathway | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 018651. | |||
| REF 2 | Clinical pipeline report, company report or official report of INSYS Therapeutics. | |||
| REF 3 | Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009 Feb;156(3):397-411. | |||
| REF 4 | Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab. 2009 Feb;23(1):133-44. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

