Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0PS6X
|
|||
| Former ID |
DNCL001914
|
|||
| Drug Name |
Mega-3-acid ethyl esters
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Hypertriglyceridemia [ICD-11: 5C80.1; ICD-10: E78.1, E78.3; ICD-9: 272.1, 427] | Approved | [1], [2] | |
| Company |
GlaxoSmithKline
|
|||
| Structure |
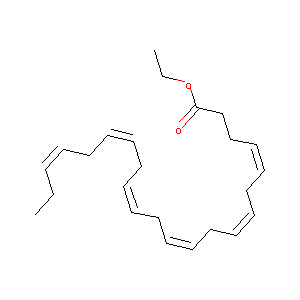 |
Download2D MOL |
||
| Formula |
C46H70O4
|
|||
| Canonical SMILES |
CCC=CCC=CCC=CCC=CCC=CCCCC(=O)OCC.CCC=CCC=CCC=CCC=CCC=CCC=CCCC(=O)OCC
|
|||
| InChI |
1S/C24H36O2.C22H34O2/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23-24(25)26-4-2;1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24-4-2/h5-6,8-9,11-12,14-15,17-18,20-21H,3-4,7,10,13,16,19,22-23H2,1-2H3;5-6,8-9,11-12,14-15,17-18H,3-4,7,10,13,16,19-21H2,1-2H3/b6-5-,9-8-,12-11-,15-14-,18-17-,21-20-;6-5-,9-8-,12-11-,15-14-,18-17-
|
|||
| InChIKey |
DTMGIJFHGGCSLO-FIAQIACWSA-N
|
|||
| CAS Number |
CAS 861006-80-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | 2004 approvals: the demise of the blockbuster. Nat Rev Drug Discov. 2005 Feb;4(2):93-4. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

