Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Q1IT
|
|||
| Former ID |
DNCL002794
|
|||
| Drug Name |
Aclidinium/formoterol
|
|||
| Synonyms |
(S,S)-Formoterol; CHEBI:63081; (S,S)-N-[2-hydroxy-5-[1-hydroxy-2-[[2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formaldehyde; NCGC00025167-01; 67346-48-9; Tocris-1448; AC1MHY5S; ZINC856
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Chronic obstructive pulmonary disease [ICD-11: CA22; ICD-10: J44, J44.9] | Phase 4 | [1], [2] | |
| Company |
Almirall
|
|||
| Structure |
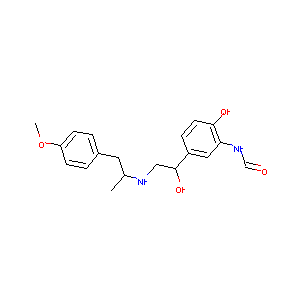 |
Download2D MOL |
||
| Formula |
C19H24N2O4
|
|||
| Canonical SMILES |
CC(CC1=CC=C(C=C1)OC)NCC(C2=CC(=C(C=C2)O)NC=O)O
|
|||
| InChI |
1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22)/t13-,19+/m0/s1
|
|||
| InChIKey |
BPZSYCZIITTYBL-ORAYPTAESA-N
|
|||
| CAS Number |
CAS 73573-87-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| ChEBI ID |
CHEBI:63081
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Duaklir Genuair approved in the European Union for chronic obstructive pulmonary disease | |||
| REF 2 | ClinicalTrials.gov (NCT02429765) Effect of Aclidinium/Formoterol on Nighttime Lung Function and Morning Symptoms in Chronic Obstructive Pulmonary Disease. U.S. National Institutes of Health. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

